Deposition Date
2021-05-15
Release Date
2022-03-16
Last Version Date
2025-05-14
Entry Detail
PDB ID:
7MW3
Keywords:
Title:
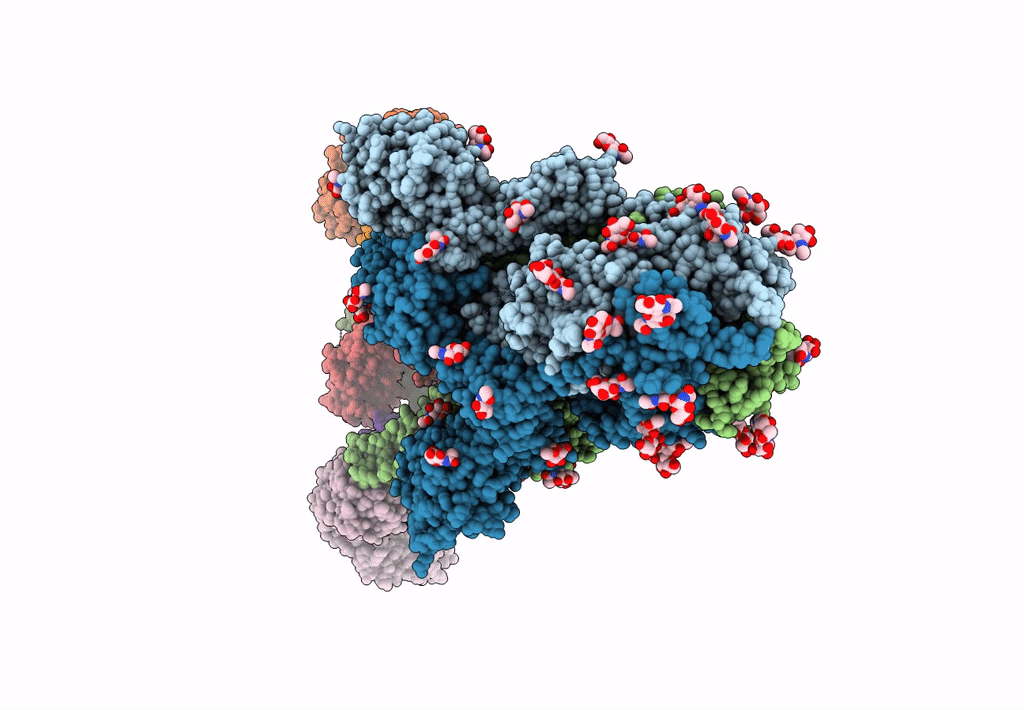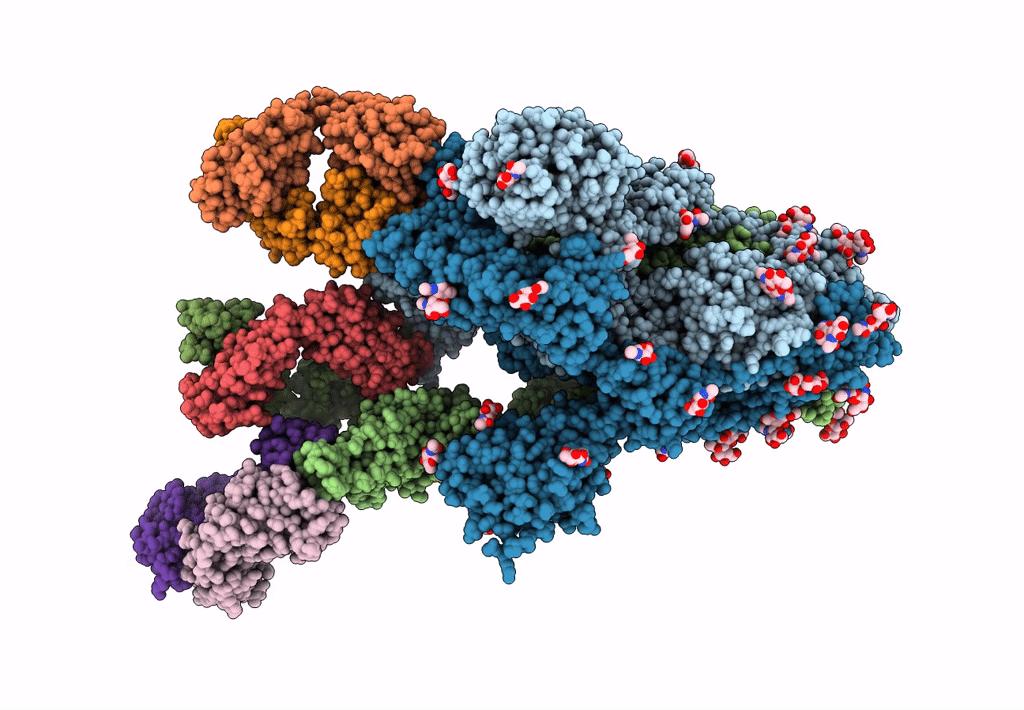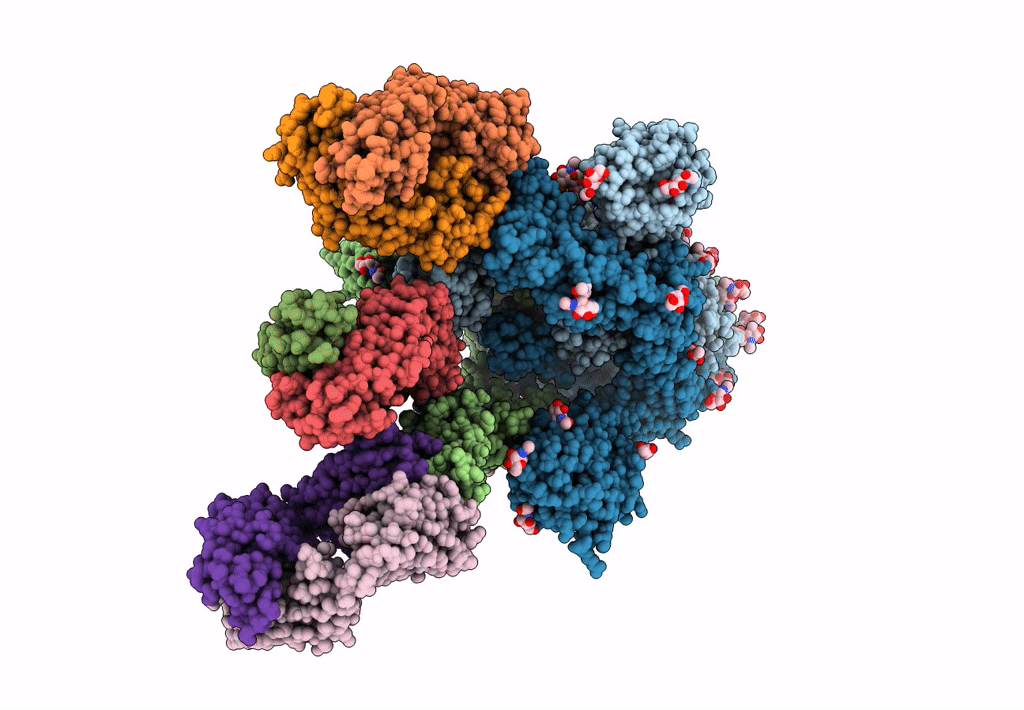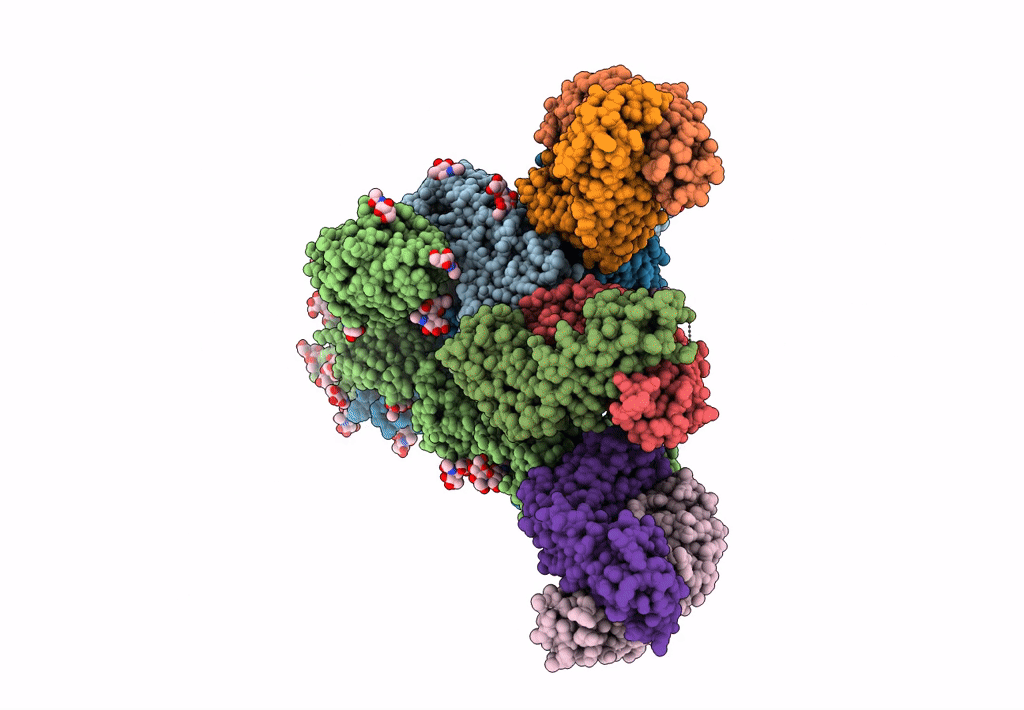
Structure of the SARS-CoV-2 Spike trimer with two RBDs down in complex with the Fab fragment of human neutralizing antibody clone 6
Biological Source:
Source Organism(s):
Homo sapiens (Taxon ID: 9606)
Severe acute respiratory syndrome coronavirus 2 (Taxon ID: 2697049)
Severe acute respiratory syndrome coronavirus 2 (Taxon ID: 2697049)
Expression System(s):
Method Details:
Experimental Method:
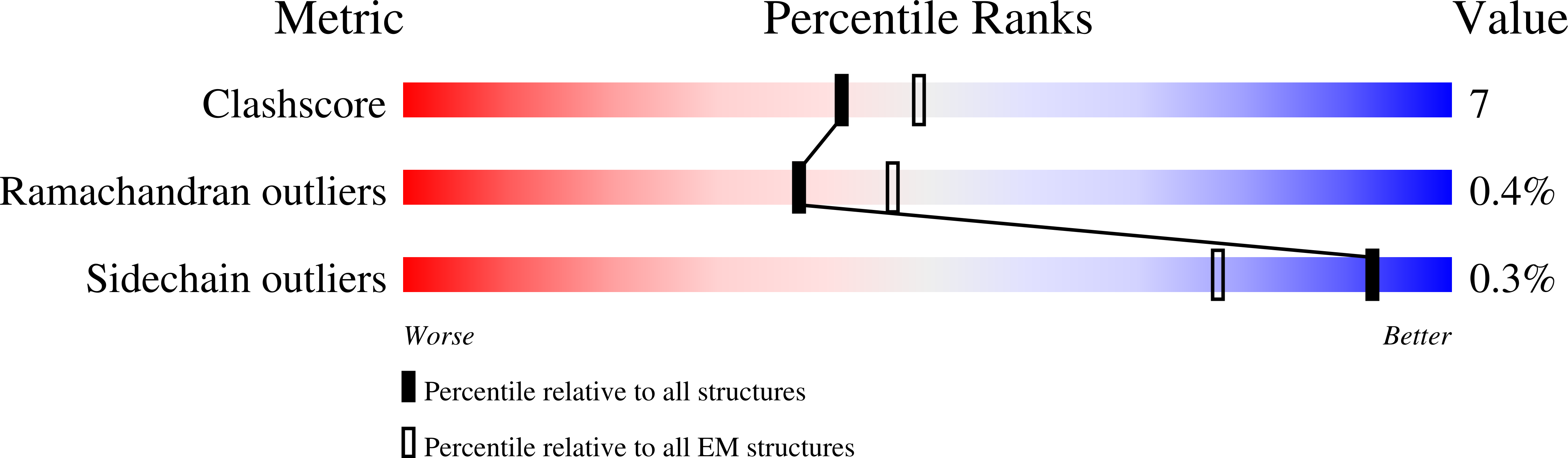
Resolution:
3.15 Å
Aggregation State:
PARTICLE
Reconstruction Method:
SINGLE PARTICLE