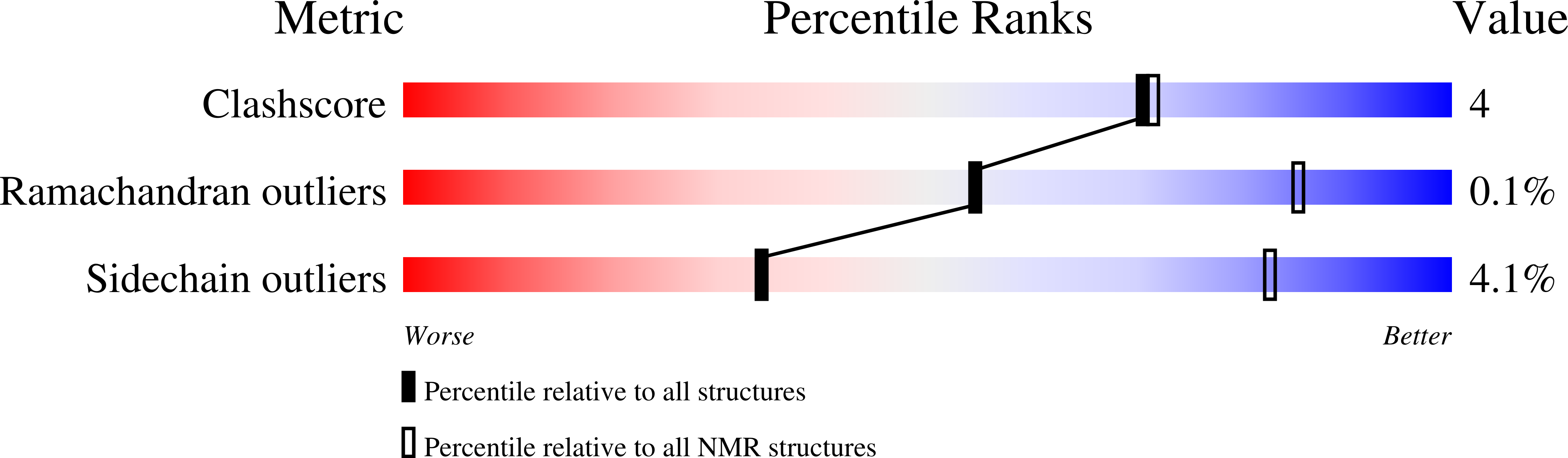Deposition Date
2021-05-14
Release Date
2021-12-22
Last Version Date
2024-05-15
Entry Detail
PDB ID:
7MU9
Keywords:
Title:
Solution NMR structure of the XVIPCD region from the T4SS effector X-Tfe(XAC2609) from Xanthomonas citri
Biological Source:
Source Organism:
Xanthomonas axonopodis pv. citri (strain 306) (Taxon ID: 190486)
Host Organism:
Method Details:
Experimental Method:
Conformers Calculated:
50
Conformers Submitted:
20
Selection Criteria:
structures with the lowest energy