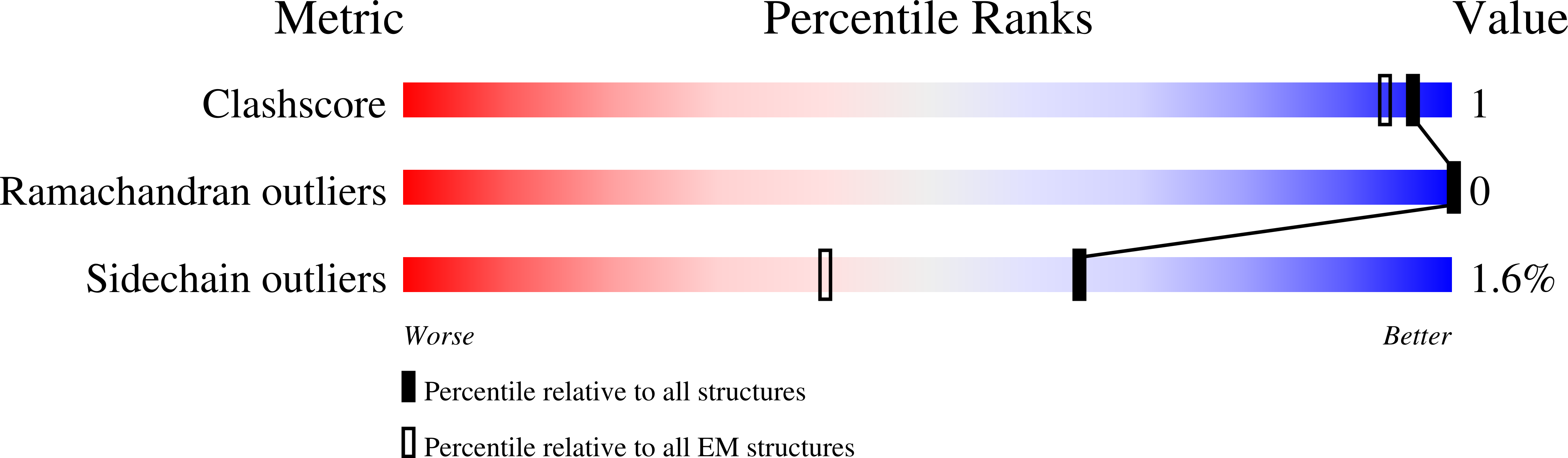Deposition Date
2021-03-25
Release Date
2022-04-06
Last Version Date
2024-10-16
Entry Detail
PDB ID:
7M69
Keywords:
Title:
E1435Q Ycf1 mutant in inward-facing wide conformation
Biological Source:
Source Organism(s):
Saccharomyces cerevisiae S288C (Taxon ID: 559292)
Expression System(s):
Method Details:
Experimental Method:
Resolution:
3.42 Å
Aggregation State:
PARTICLE
Reconstruction Method:
SINGLE PARTICLE