Deposition Date
2021-03-18
Release Date
2022-01-05
Last Version Date
2024-05-29
Entry Detail
PDB ID:
7M33
Keywords:
Title:
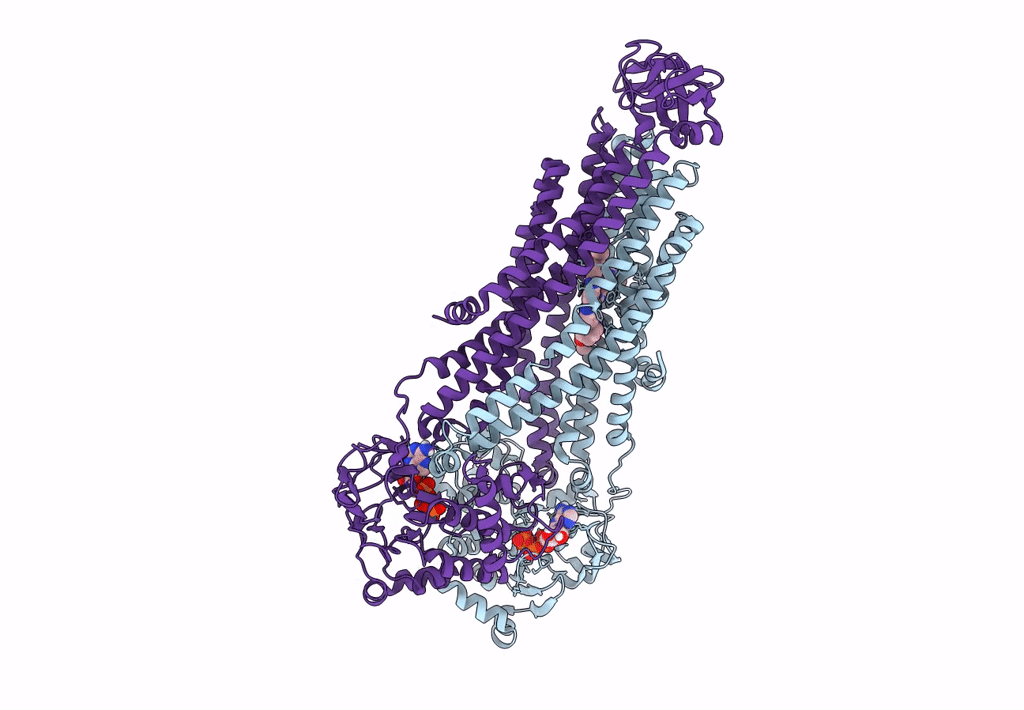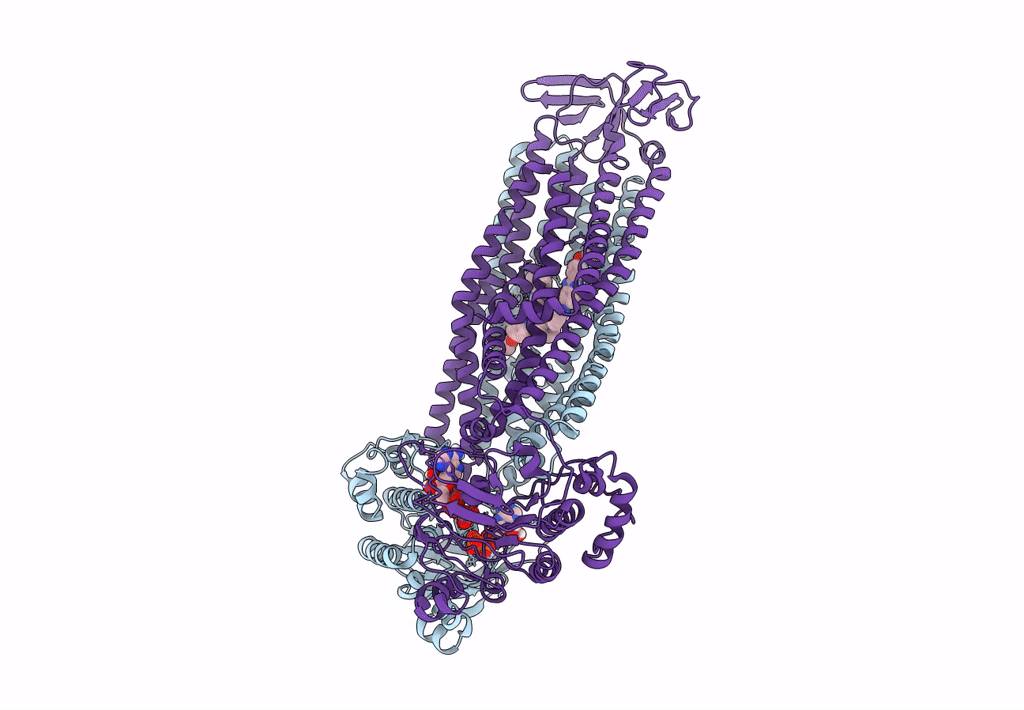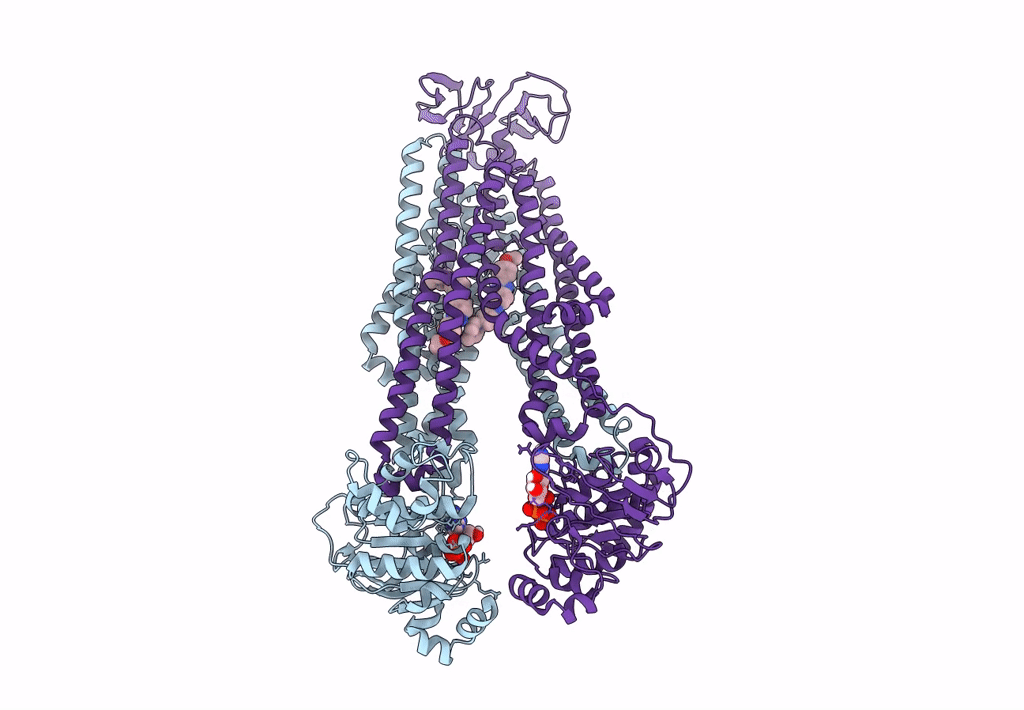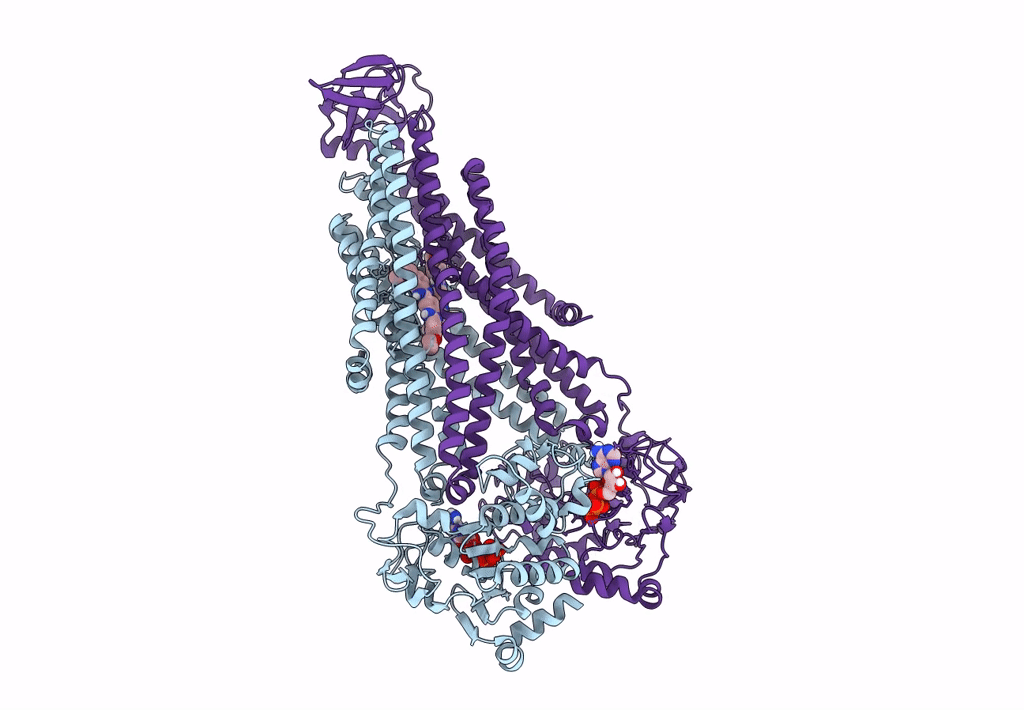
The structure of Bacillus subtilis BmrCD in the inward-facing conformation bound to Hoechst-33342 and ATP
Biological Source:
Source Organism(s):
Bacillus subtilis subsp. subtilis str. 168 (Taxon ID: 224308 )
Expression System(s):
Method Details:
Experimental Method:
Resolution:
3.55 Å
Aggregation State:
PARTICLE
Reconstruction Method:
SINGLE PARTICLE


