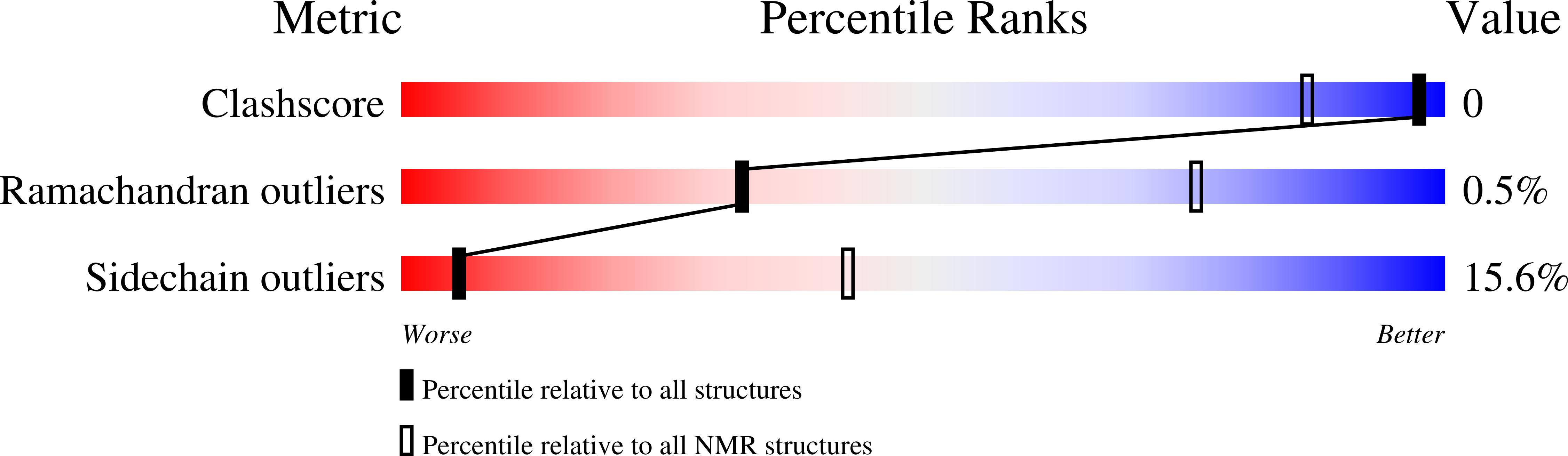
Deposition Date
2021-03-12
Release Date
2022-01-19
Last Version Date
2024-05-15
Entry Detail
PDB ID:
7M1E
Keywords:
Title:
Structural and functional studies about scorpine showed the presence of blocking channel and cytolytic activities as well as two different structural domains
Biological Source:
Source Organism(s):
Pandinus imperator (Taxon ID: 55084)
Expression System(s):
Method Details:
Experimental Method:
Conformers Calculated:
500
Conformers Submitted:
20
Selection Criteria:
structures with the lowest energy