Deposition Date
2021-01-13
Release Date
2021-07-07
Last Version Date
2024-11-20
Entry Detail
PDB ID:
7LDL
Keywords:
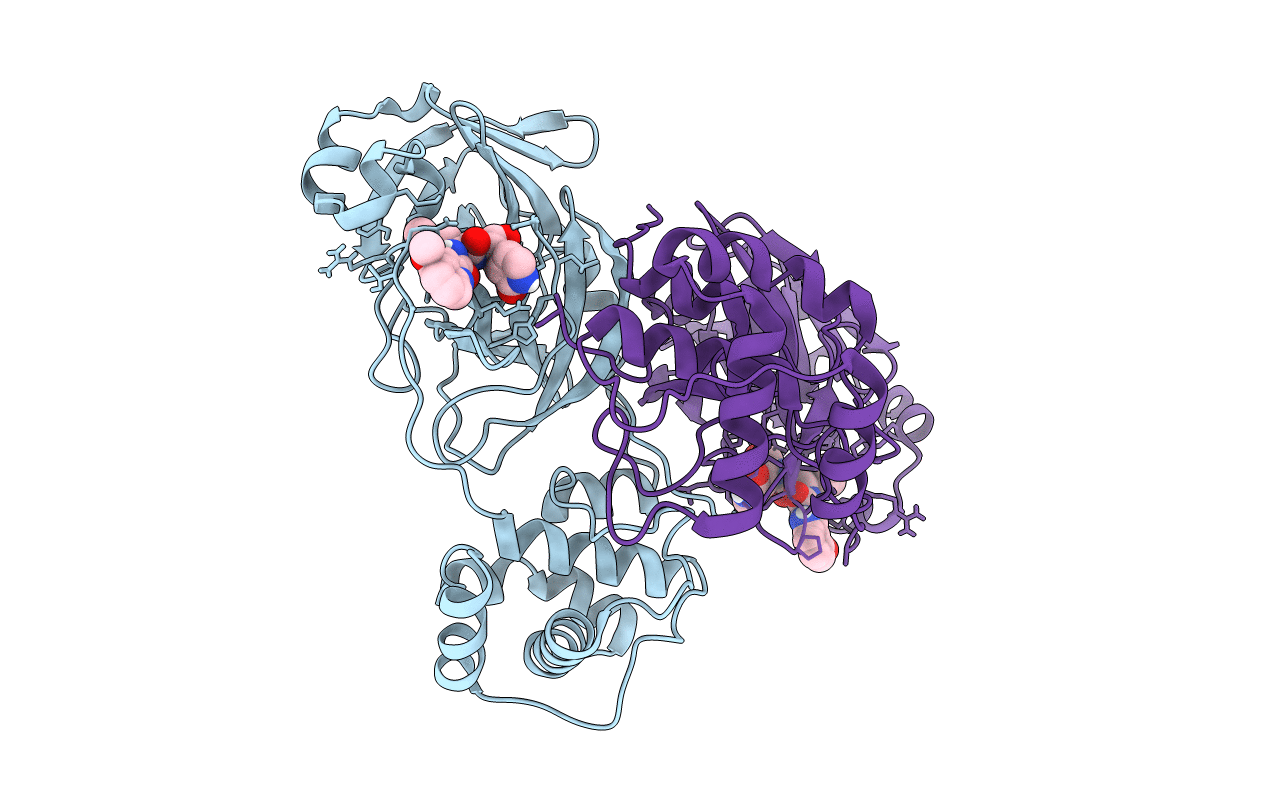
Title:
Improved Feline Drugs as SARS-CoV-2 Mpro Inhibitors: Structure-Activity Studies & Micellar Solubilization for Enhanced Bioavailability
Biological Source:
Source Organism(s):
Expression System(s):
Method Details:
Experimental Method:
Resolution:
2.00 Å
R-Value Free:
0.26
R-Value Work:
0.20
R-Value Observed:
0.20
Space Group:
P 1


