Deposition Date
2020-12-15
Release Date
2021-07-28
Last Version Date
2023-10-18
Entry Detail
PDB ID:
7L1Y
Keywords:
Title:
Unlocking the structural features for the exo-xylobiosidase activity of an unusual GH11 member identified in a compost-derived consortium-xylobiose complex
Biological Source:
Source Organism(s):
unidentified (Taxon ID: 32644)
Expression System(s):
Method Details:
Experimental Method:
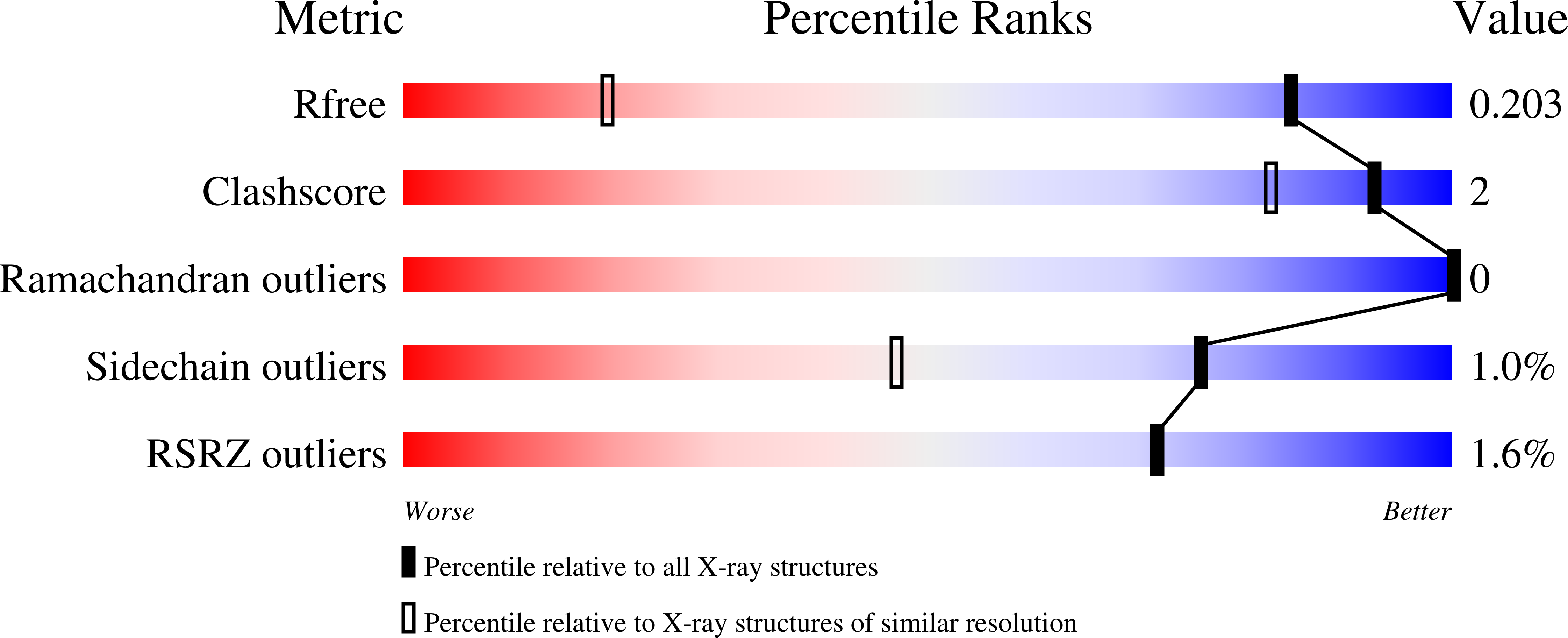
Resolution:
1.20 Å
R-Value Free:
0.20
R-Value Work:
0.17
R-Value Observed:
0.18
Space Group:
P 43 21 2