Deposition Date
2020-11-18
Release Date
2021-05-19
Last Version Date
2023-10-18
Entry Detail
PDB ID:
7KR6
Keywords:
Title:
Glycoside hydrolase family 16 endo-glucanase from Bacteroides ovatus in complex with G4G3G-2F-DNP
Biological Source:
Source Organism:
Bacteroides ovatus (Taxon ID: 28116)
Host Organism:
Method Details:
Experimental Method:
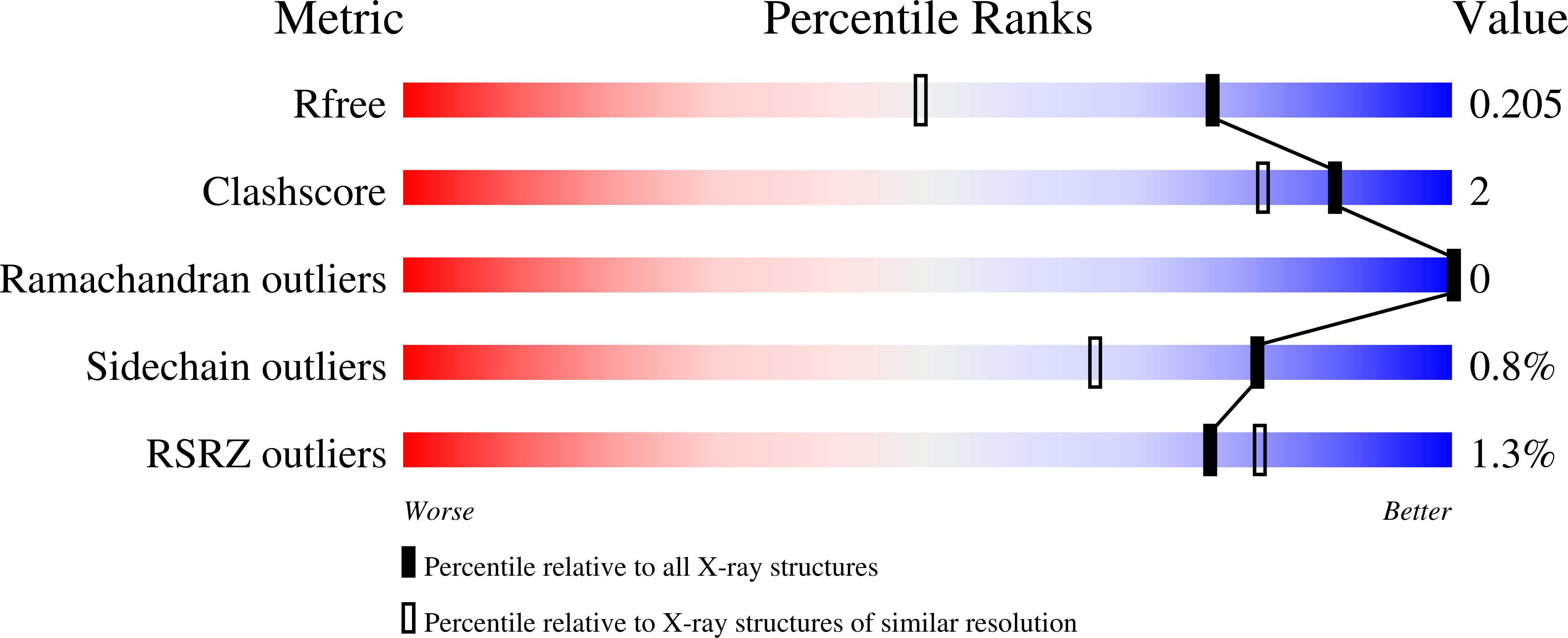
Resolution:
1.56 Å
R-Value Free:
0.19
R-Value Work:
0.17
Space Group:
P 1 21 1