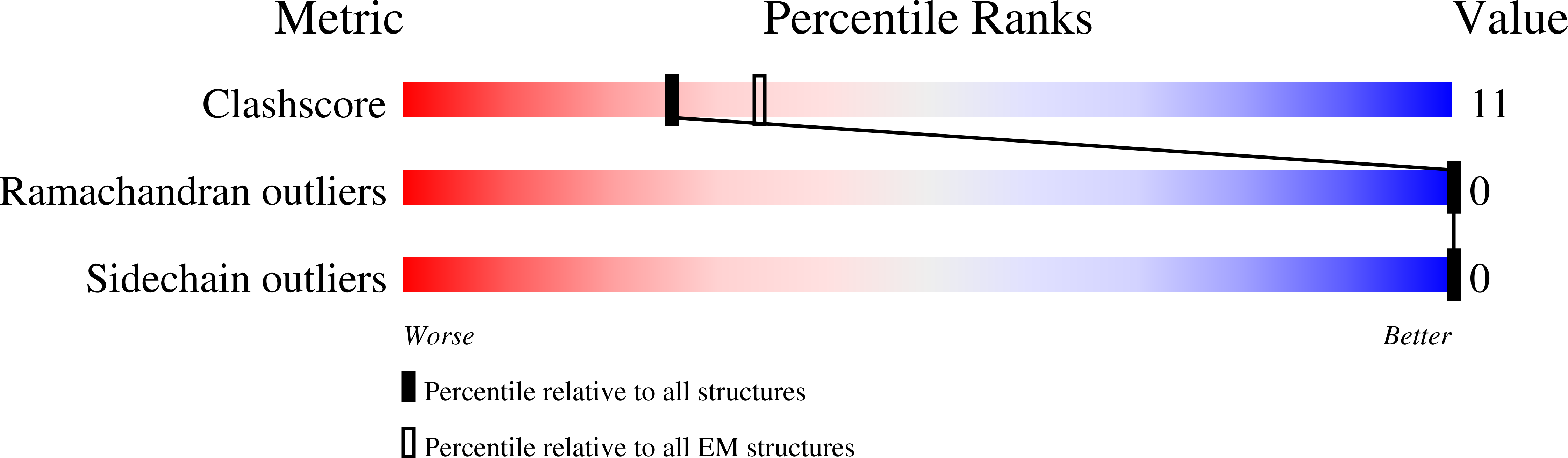
Deposition Date
2020-11-18
Release Date
2020-12-09
Last Version Date
2025-05-21
Entry Detail
PDB ID:
7KR5
Keywords:
Title:
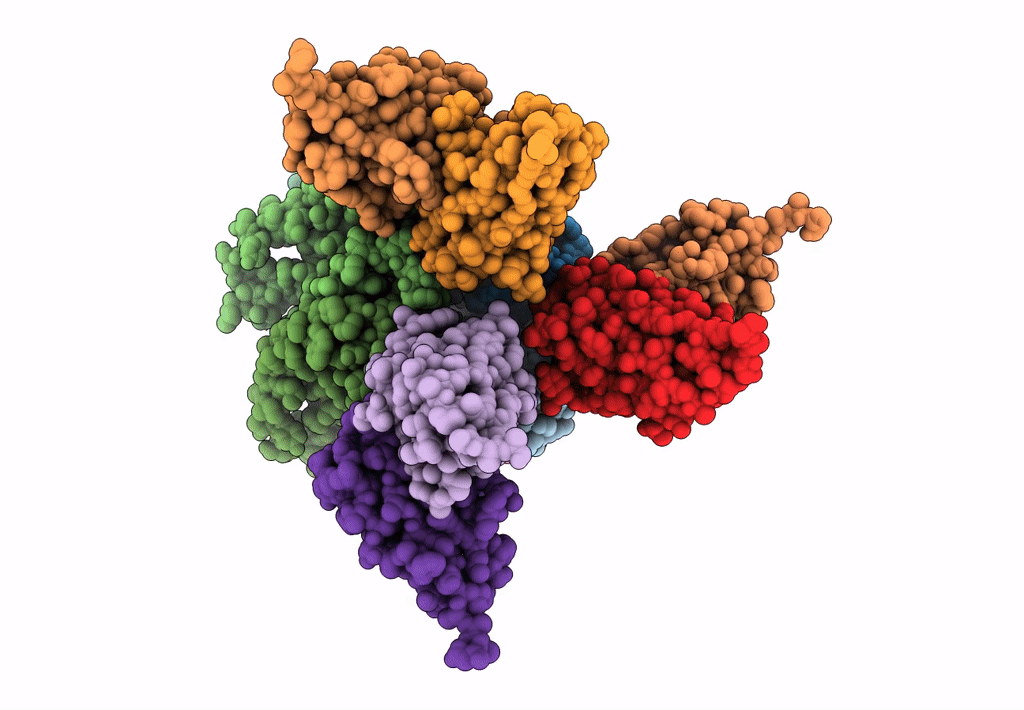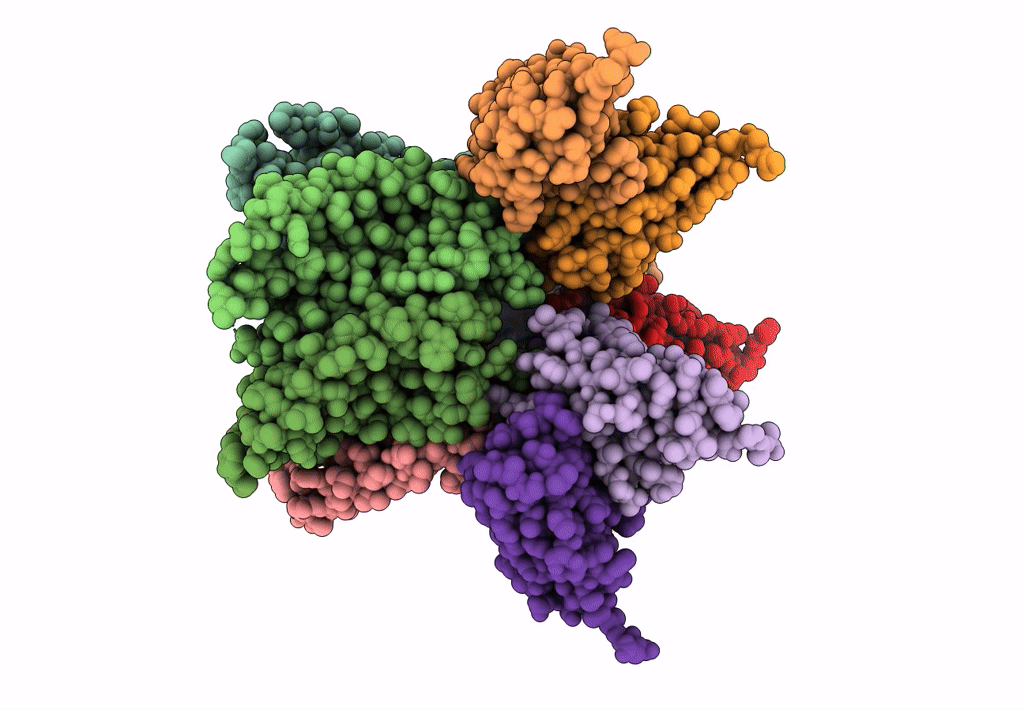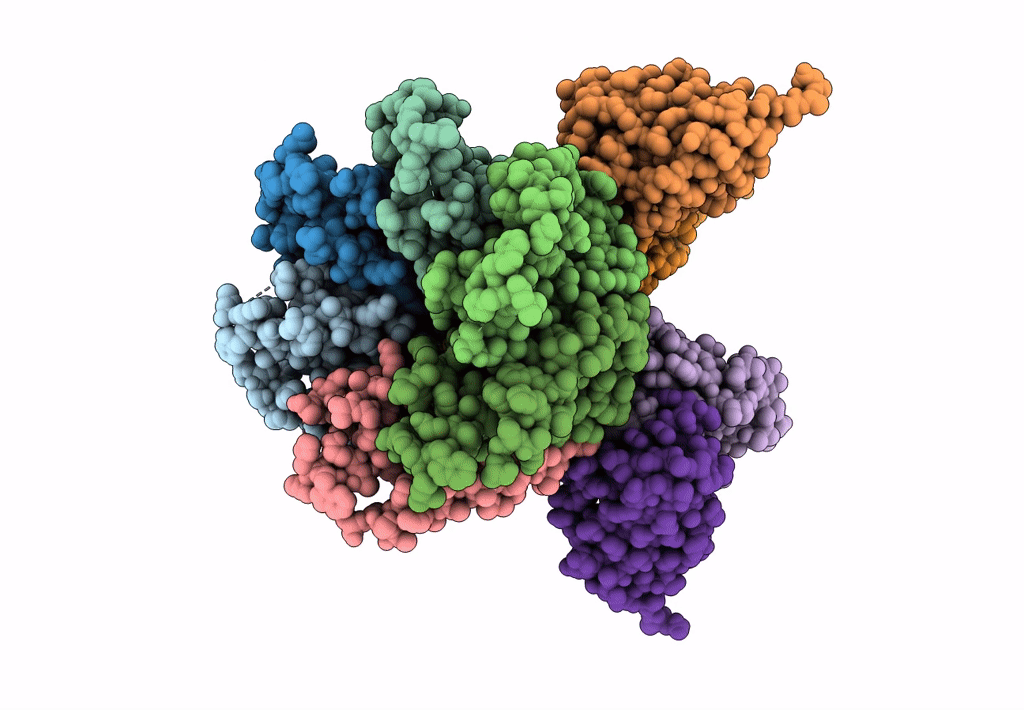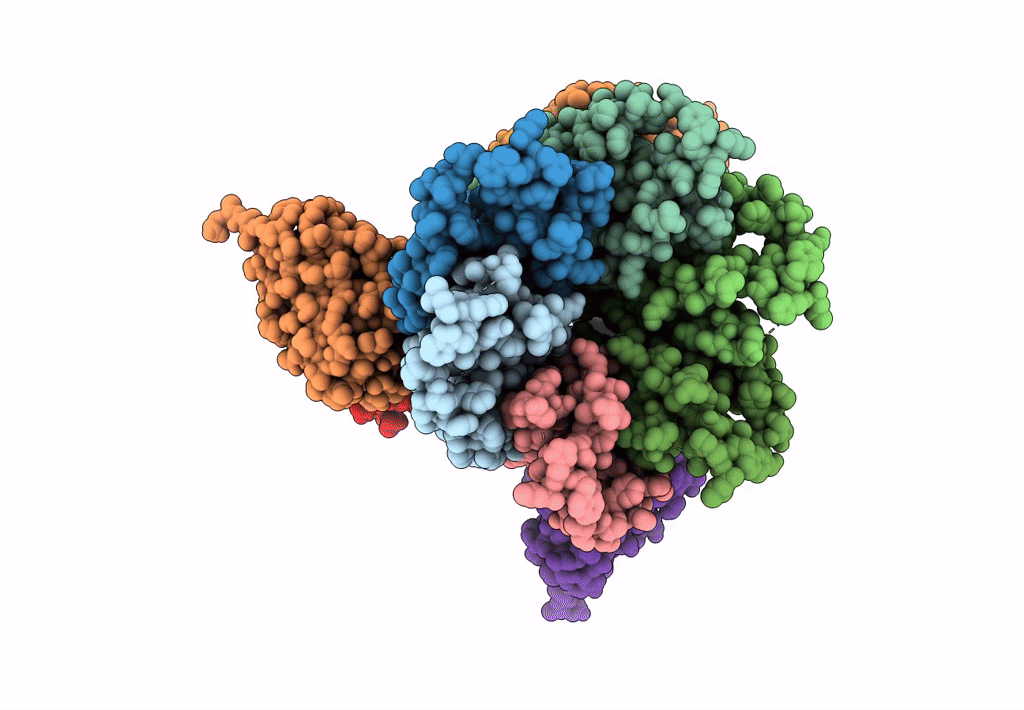
Cryo-EM structure of the CRAC channel Orai in an open conformation; H206A gain-of-function mutation in complex with an antibody
Biological Source:
Source Organism:
Drosophila melanogaster (Taxon ID: 7227)
Mus musculus (Taxon ID: 10090)
Mus musculus (Taxon ID: 10090)
Host Organism:
Method Details:
Experimental Method:
Resolution:
3.30 Å
Aggregation State:
PARTICLE
Reconstruction Method:
SINGLE PARTICLE