Deposition Date
2020-11-09
Release Date
2021-01-27
Last Version Date
2024-11-20
Entry Detail
PDB ID:
7KOT
Keywords:
Title:
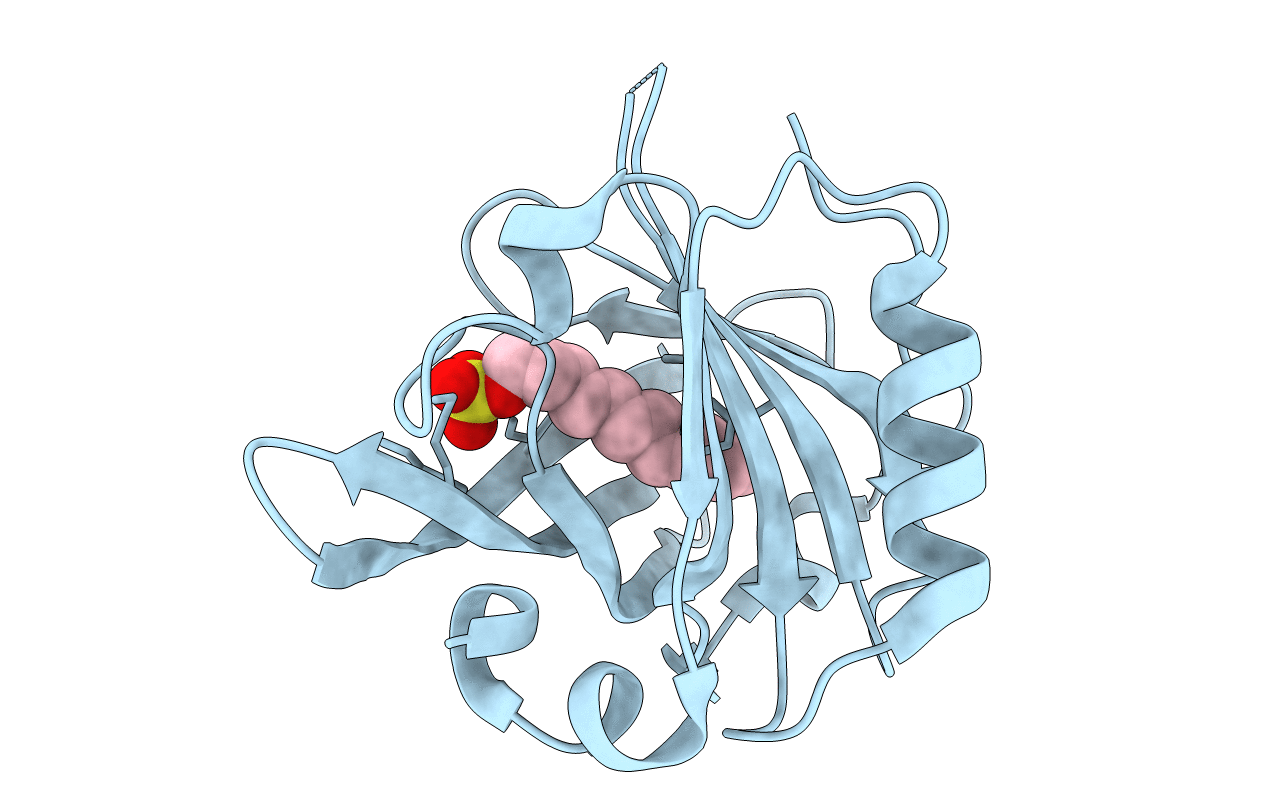
Energetic and structural effects of the Tanford transition on the ligand recognition of bovine Beta-lactoglobulin
Biological Source:
Source Organism(s):
Bos taurus (Taxon ID: 9913)
Method Details:
Experimental Method:
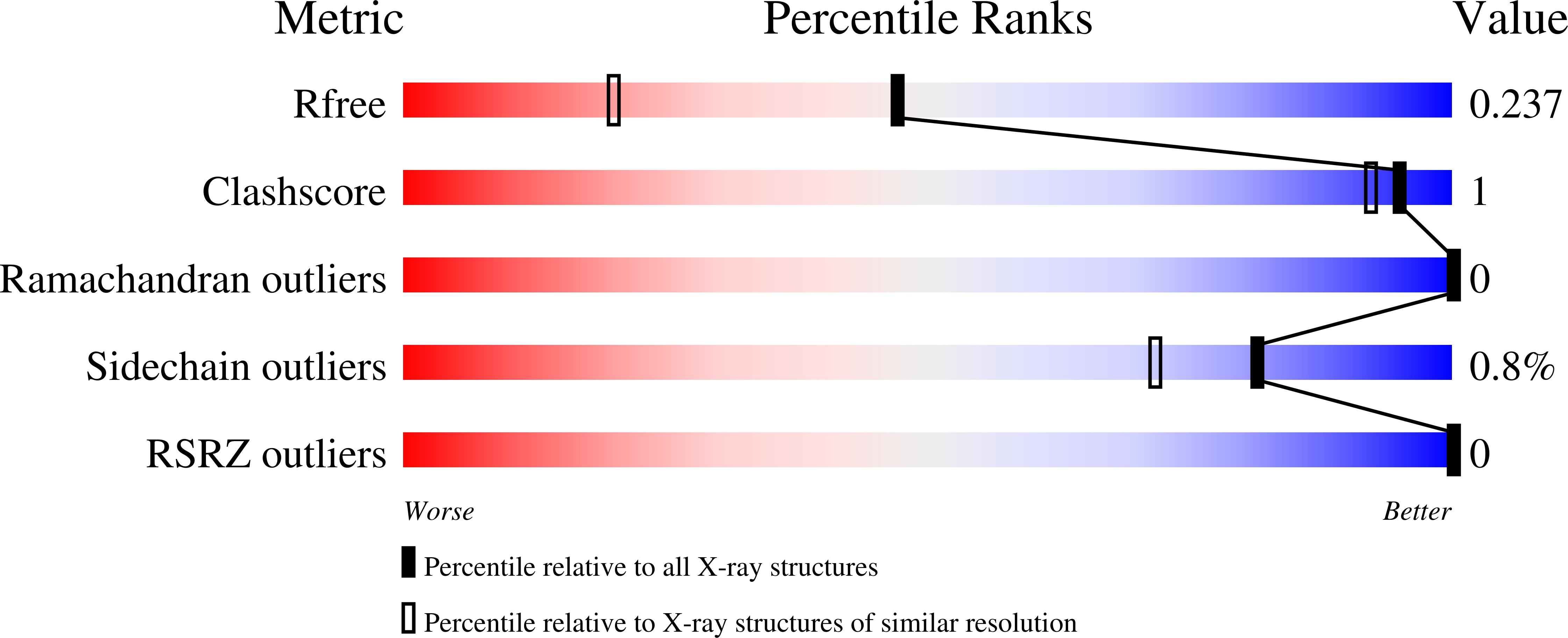
Resolution:
1.74 Å
R-Value Free:
0.22
R-Value Work:
0.19
Space Group:
P 32 2 1