Deposition Date
2020-11-02
Release Date
2021-07-07
Last Version Date
2024-11-06
Entry Detail
PDB ID:
7KMD
Keywords:
Title:
Crystal structure of a HIV-1 clade C isolate Du172.17 HR1.R4.664 Env trimer in complex with human Fabs PGT124 and 35O22
Biological Source:
Source Organism(s):
Homo sapiens (Taxon ID: 9606)
Human immunodeficiency virus 1 (Taxon ID: 11676)
Human immunodeficiency virus 1 (Taxon ID: 11676)
Expression System(s):
Method Details:
Experimental Method:
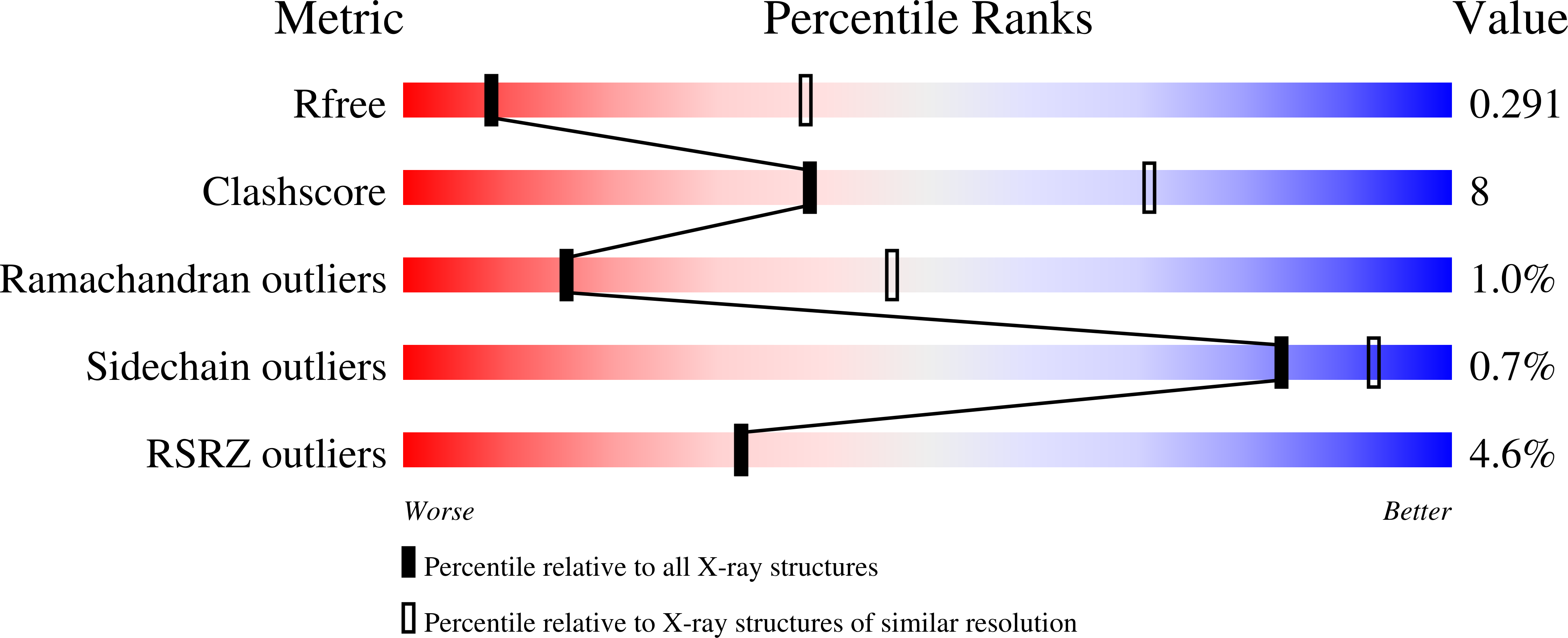
Resolution:
3.39 Å
R-Value Free:
0.29
R-Value Work:
0.24
R-Value Observed:
0.24
Space Group:
P 63