Deposition Date
2020-10-08
Release Date
2021-10-06
Last Version Date
2023-10-18
Entry Detail
PDB ID:
7KD5
Keywords:
Title:
Structure of the C-terminal domain of the Menangle virus phosphoprotein (residues 329 -388), fused to MBP. Space group P212121
Biological Source:
Source Organism(s):
Serratia sp. (strain FS14) (Taxon ID: 1327989)
Menangle virus (Taxon ID: 152219)
Menangle virus (Taxon ID: 152219)
Expression System(s):
Method Details:
Experimental Method:
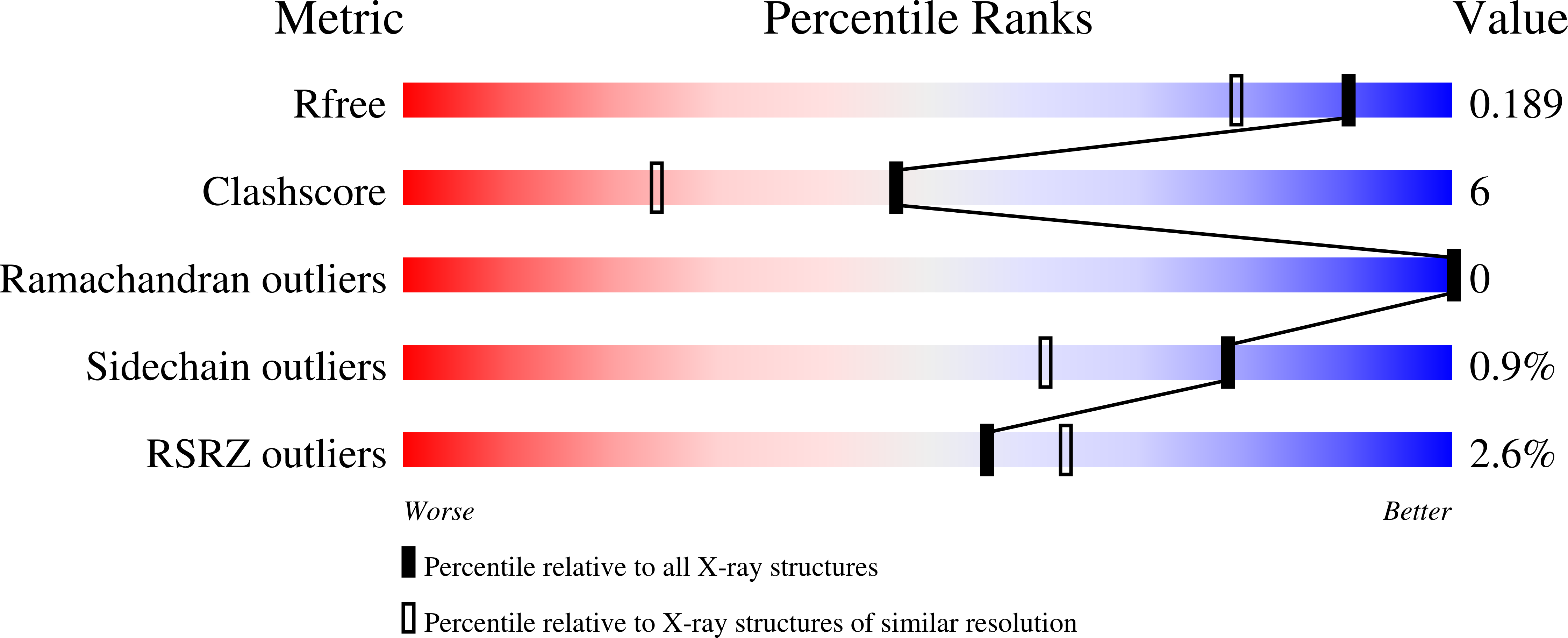
Resolution:
1.55 Å
R-Value Free:
0.18
R-Value Work:
0.16
Space Group:
P 21 21 21