Deposition Date
2020-08-27
Release Date
2020-12-09
Last Version Date
2025-04-02
Entry Detail
PDB ID:
7JXN
Keywords:
Title:
Beta hairpin derived from Abeta17-36 with an F20Cha mutation
Biological Source:
Source Organism:
Homo sapiens (Taxon ID: 9606)
Method Details:
Experimental Method:
Resolution:
2.00 Å
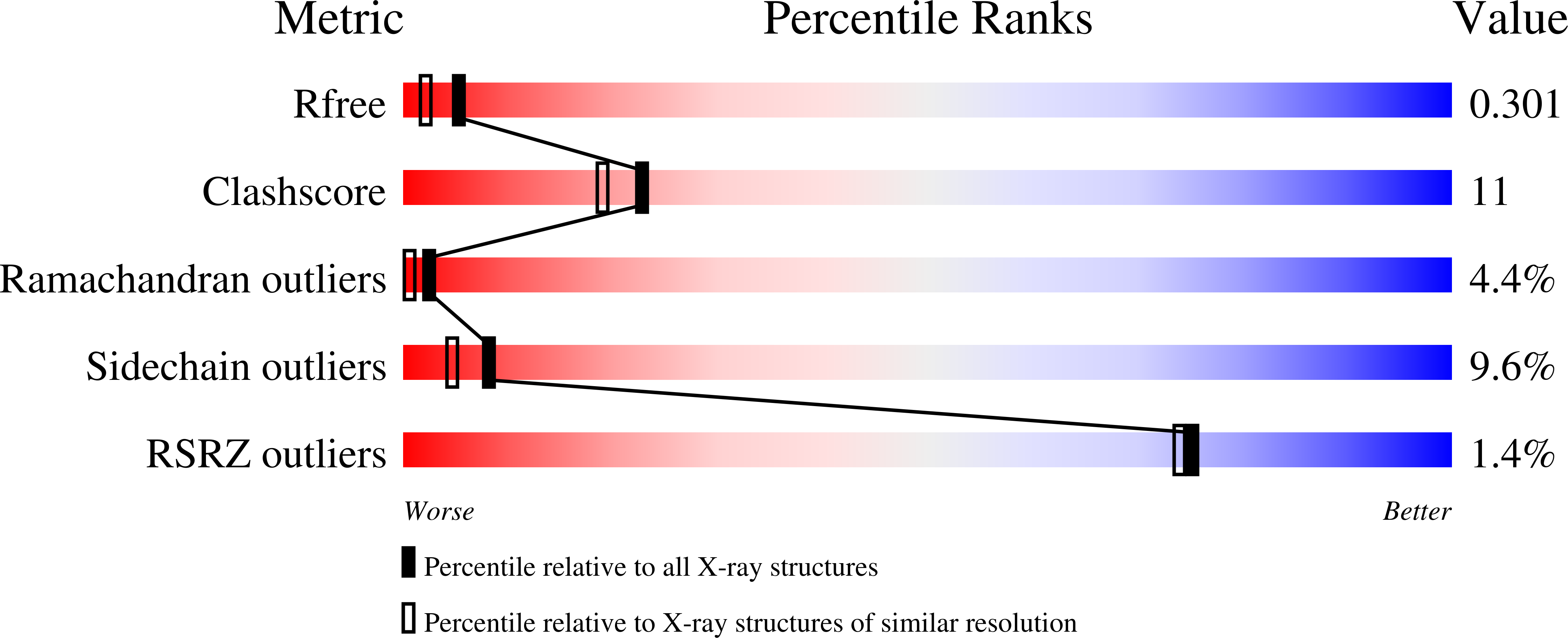
R-Value Free:
0.30
R-Value Work:
0.27
R-Value Observed:
0.28
Space Group:
P 63