Deposition Date
2020-07-27
Release Date
2021-01-27
Last Version Date
2024-10-16
Entry Detail
PDB ID:
7JJV
Keywords:
Title:
Crystal waters on the nine polyproline type II helical bundle springtail antifreeze protein from Granisotoma rainieri match the ice lattice
Biological Source:
Source Organism(s):
unclassified Entomobryomorpha (Taxon ID: 730332)
Expression System(s):
Method Details:
Experimental Method:
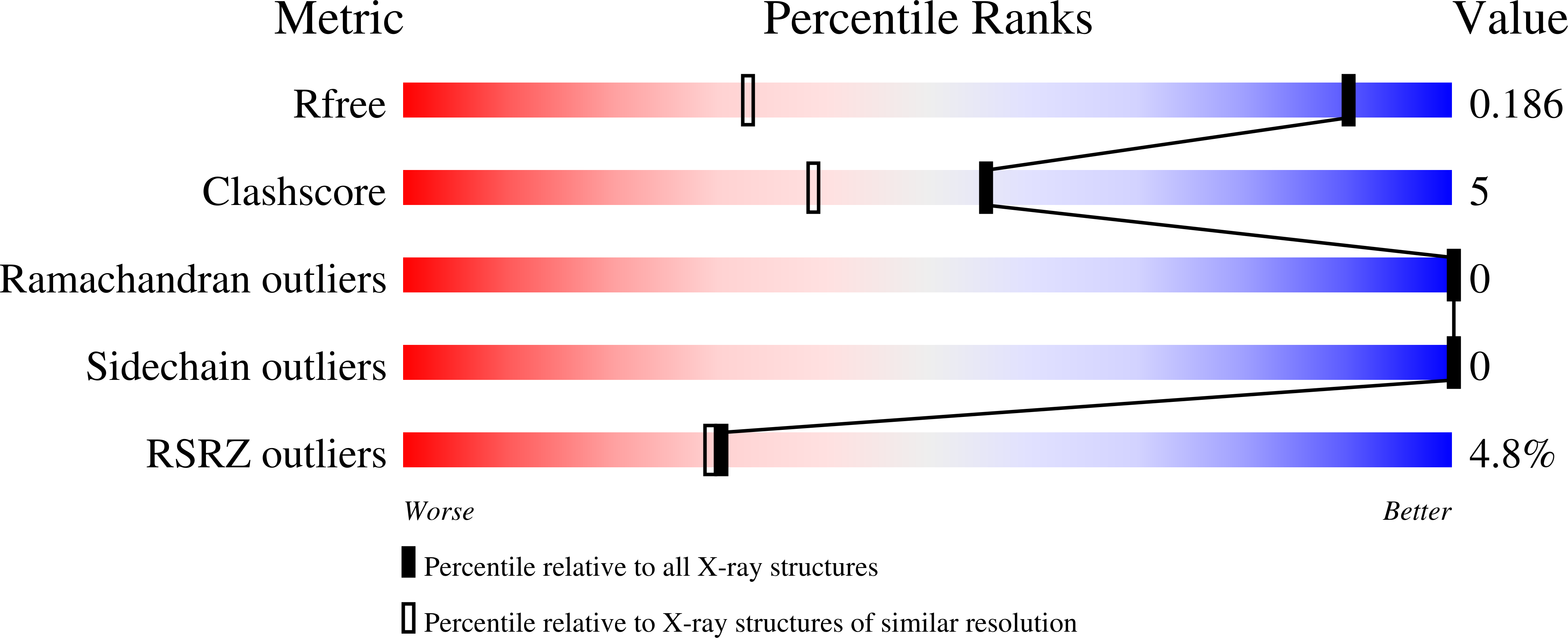
Resolution:
1.21 Å
R-Value Free:
0.18
R-Value Work:
0.14
Space Group:
P 21 21 21