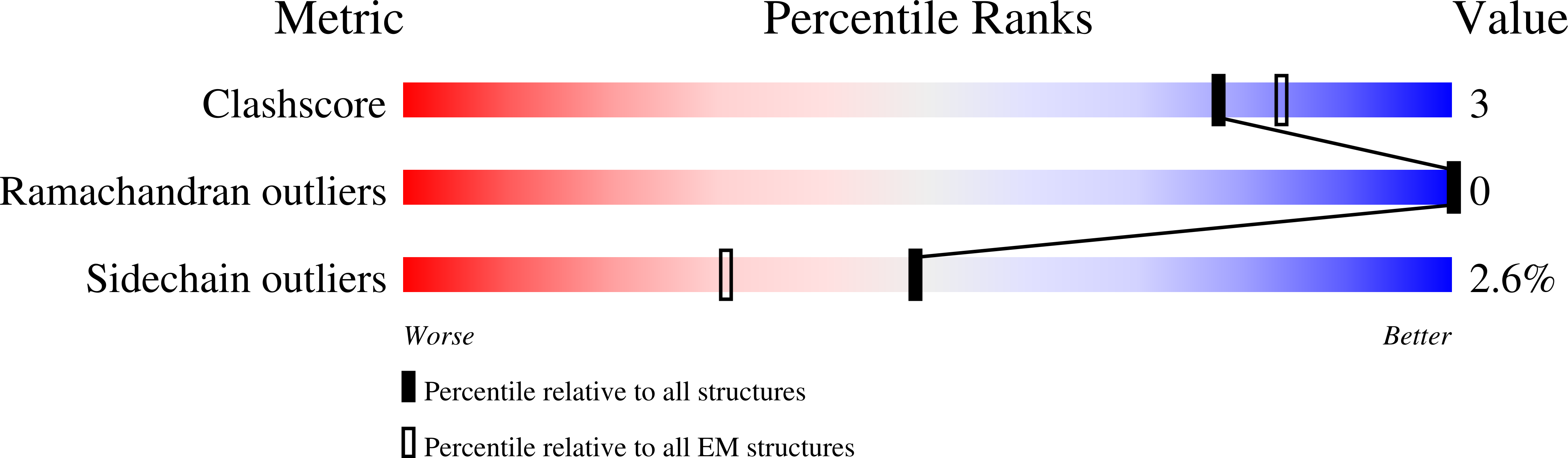
Deposition Date
2020-07-27
Release Date
2020-09-02
Last Version Date
2024-11-20
Entry Detail
PDB ID:
7JJO
Keywords:
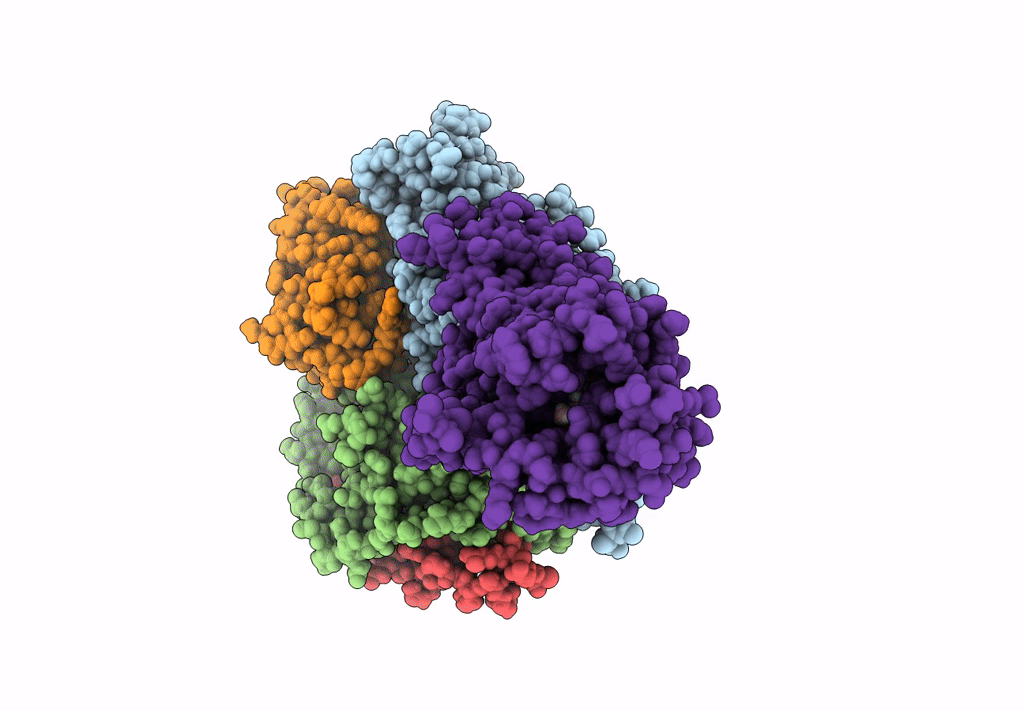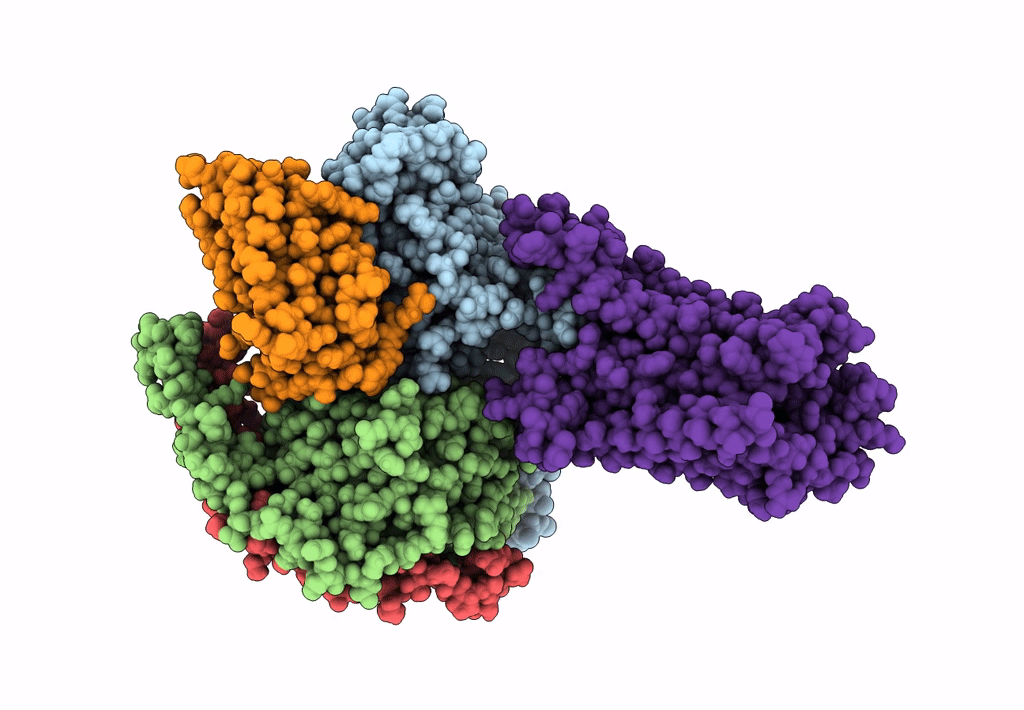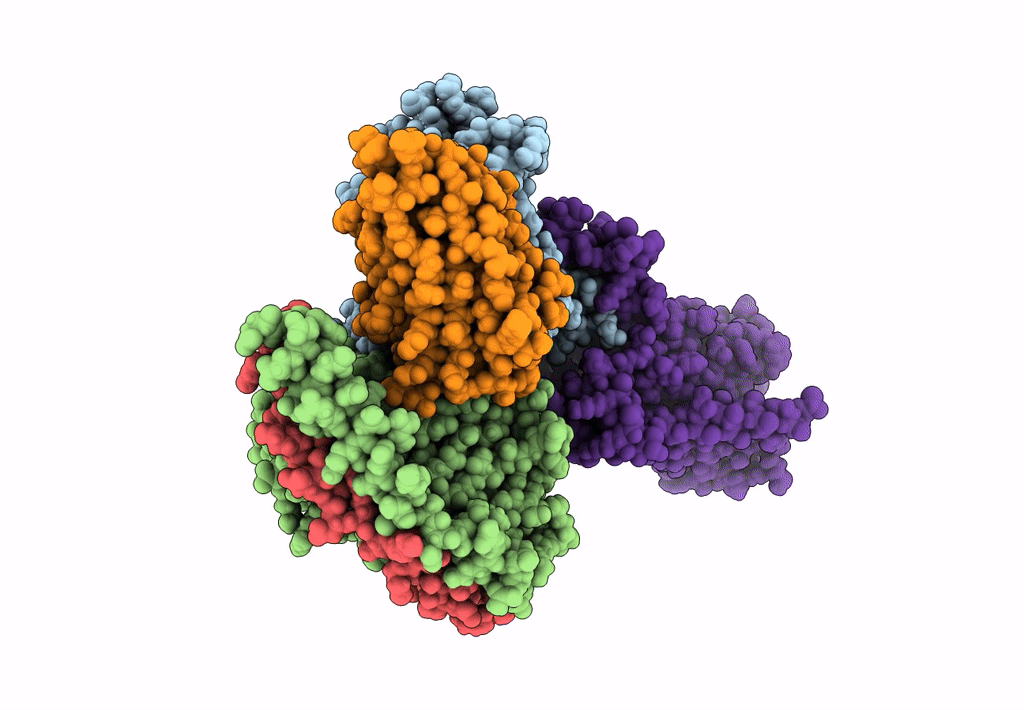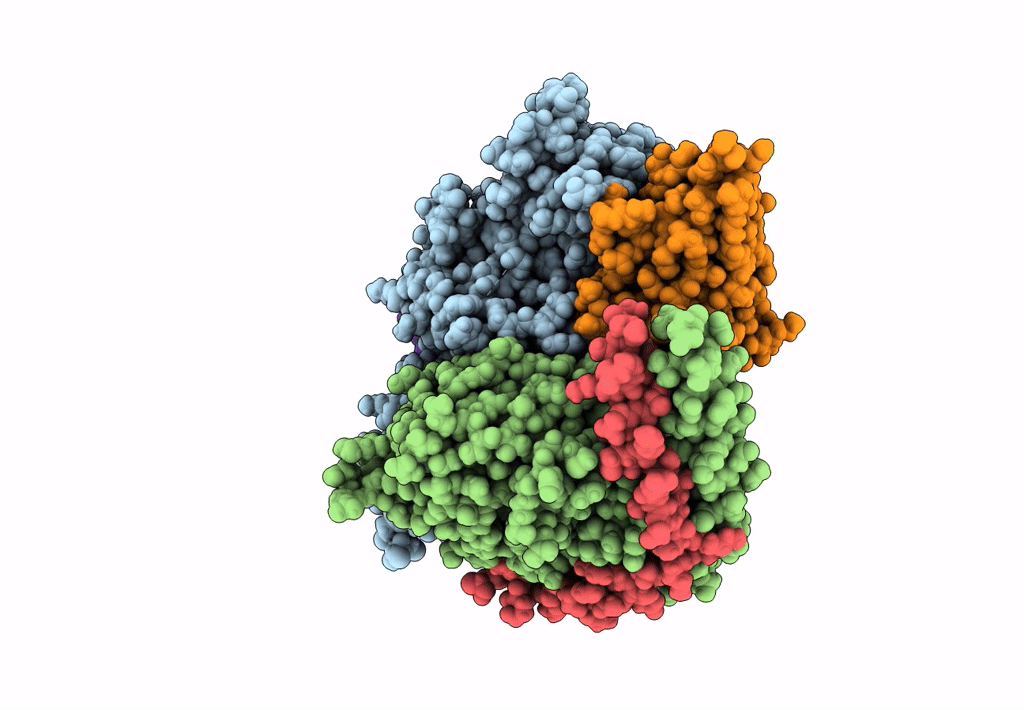
Title:
Structural Basis of the Activation of Heterotrimeric Gs-protein by Isoproterenol-bound Beta1-Adrenergic Receptor
Biological Source:
Source Organism:
Bos taurus (Taxon ID: 9913)
Lama glama (Taxon ID: 9844)
Meleagris gallopavo (Taxon ID: 9103)
Lama glama (Taxon ID: 9844)
Meleagris gallopavo (Taxon ID: 9103)
Host Organism:
Method Details:
Experimental Method:
Resolution:
2.60 Å
Aggregation State:
PARTICLE
Reconstruction Method:
SINGLE PARTICLE