Deposition Date
2023-04-27
Release Date
2023-06-14
Last Version Date
2025-08-13
Entry Detail
PDB ID:
7FW9
Keywords:
Title:
Crystal Structure of human FABP4 in complex with 2-[(3-ethoxycarbonyl-4,5,6,7-tetrahydro-1-benzothiophen-2-yl)carbamoyl]cyclopentene-1-carboxylic acid, i.e. SMILES C1CCC2=C(C1)C(=C(S2)NC(=O)C1=C(C(=O)O)CCC1)C(=O)OCC with IC50=1.1 microM
Biological Source:
Source Organism(s):
Homo sapiens (Taxon ID: 9606)
Expression System(s):
Method Details:
Experimental Method:
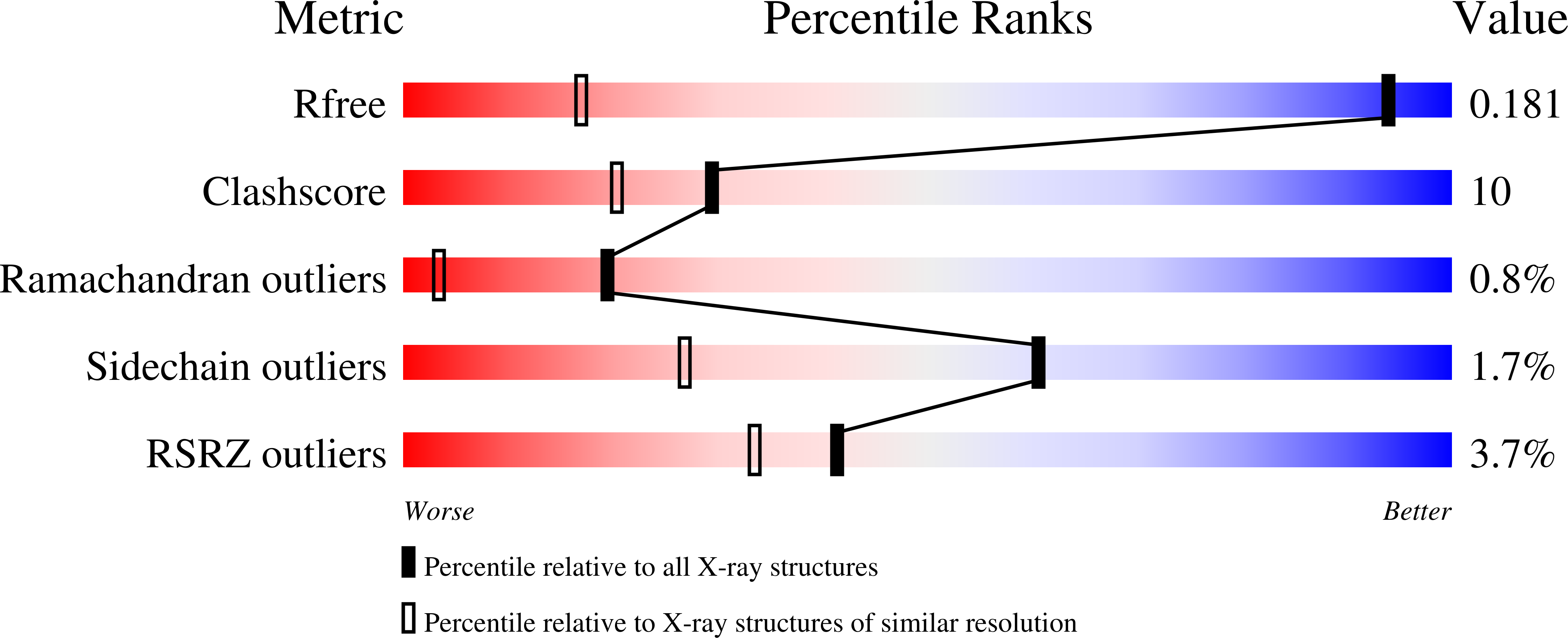
Resolution:
1.00 Å
R-Value Free:
0.17
R-Value Work:
0.15
R-Value Observed:
0.15
Space Group:
P 21 21 21