Deposition Date
2021-07-14
Release Date
2022-02-02
Last Version Date
2023-11-29
Entry Detail
PDB ID:
7FC9
Keywords:
Title:
Crystal structure of CmABCB1 in lipidic mesophase revealed by LCP-SFX
Biological Source:
Source Organism(s):
Cyanidioschyzon merolae (strain 10D) (Taxon ID: 280699)
Expression System(s):
Method Details:
Experimental Method:
Resolution:
2.20 Å
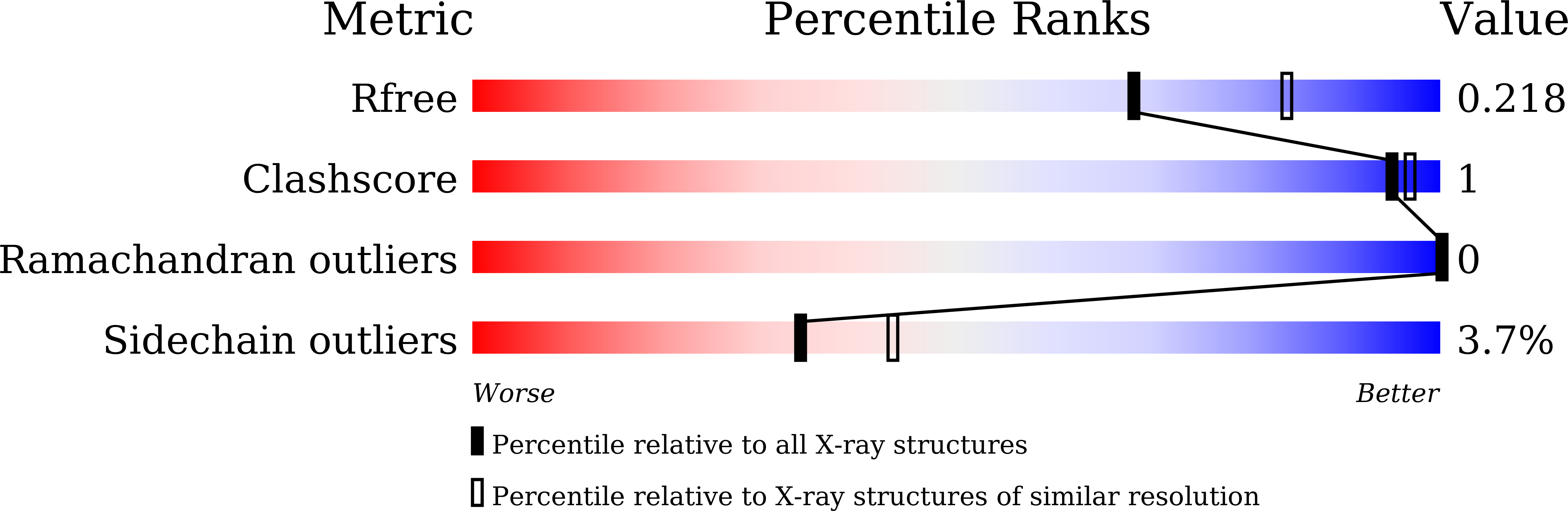
R-Value Free:
0.21
R-Value Work:
0.19
Space Group:
C 2 2 21