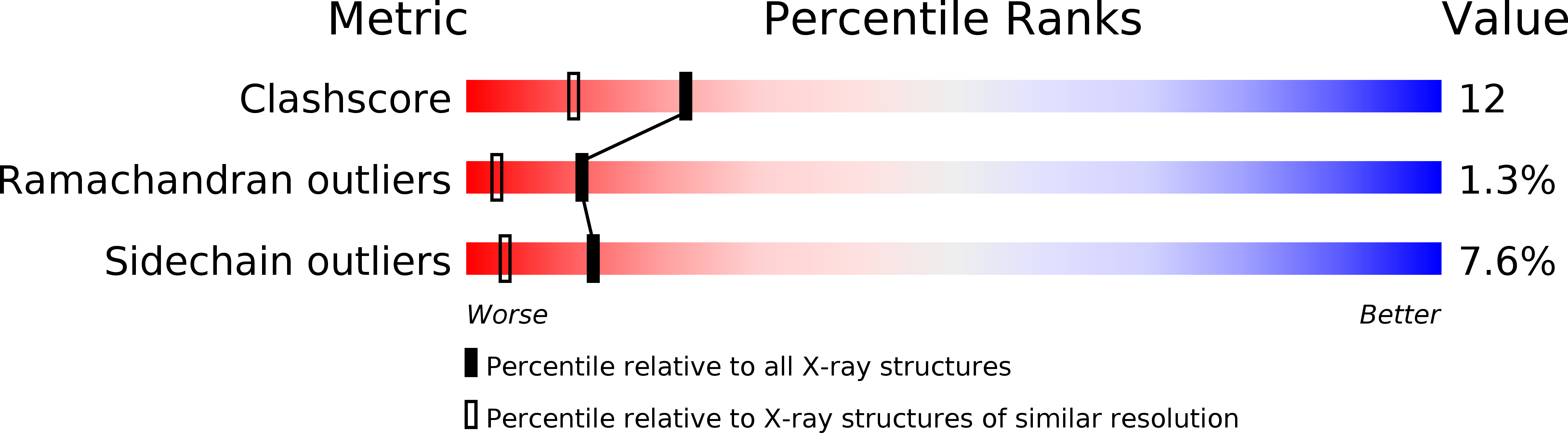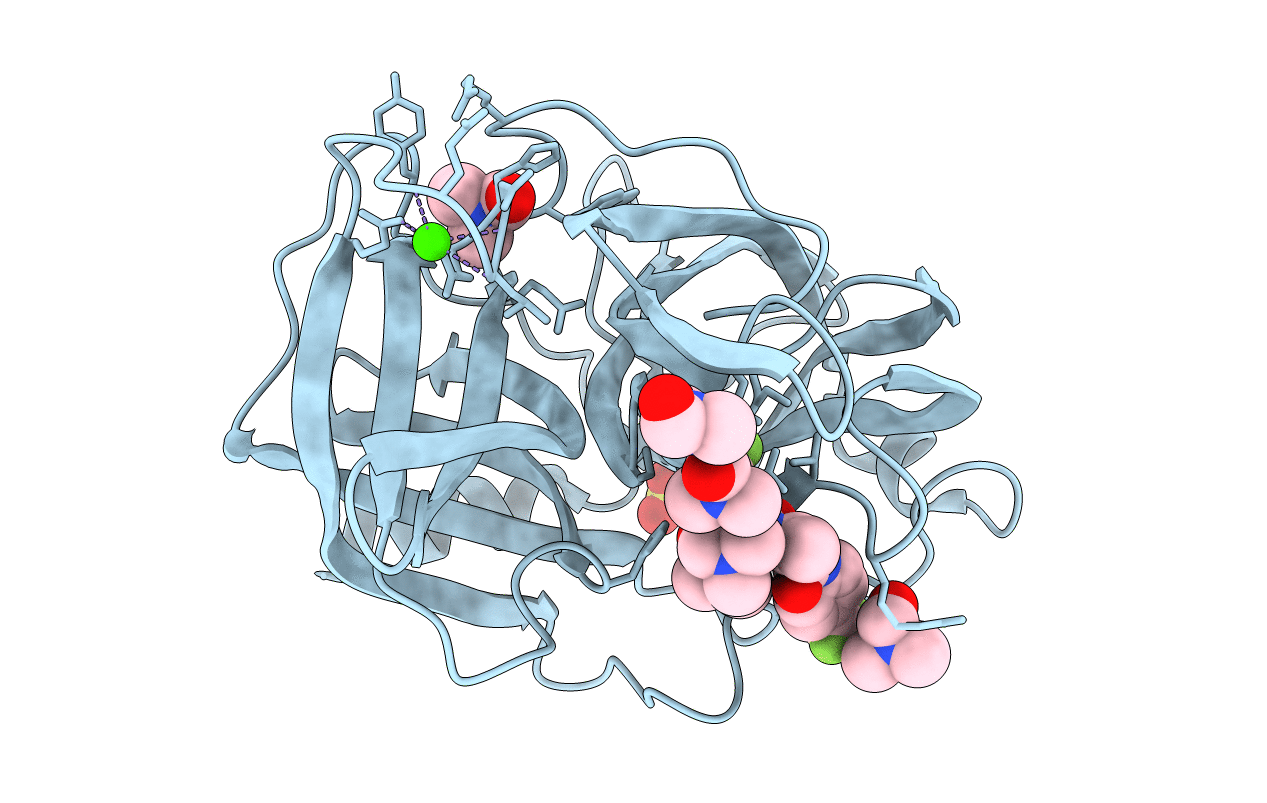
Deposition Date
1990-06-15
Release Date
1991-10-15
Last Version Date
2024-10-30
Entry Detail
PDB ID:
7EST
Keywords:
Title:
Interaction of the peptide CF3-LEU-ALA-NH-C6H4-CF3(TFLA) with porcine pancreatic elastase. X-ray studies at 1.8 Angstroms
Biological Source:
Source Organism(s):
Sus scrofa (Taxon ID: 9823)
Method Details:
Experimental Method:
Resolution:
1.80 Å
R-Value Work:
0.19
Space Group:
P 21 21 21