Deposition Date
2021-04-01
Release Date
2021-09-22
Last Version Date
2025-04-09
Entry Detail
PDB ID:
7EIZ
Keywords:
Title:
Coupling of N7-methyltransferase and 3'-5' exoribonuclease with SARS-CoV-2 polymerase reveals mechanisms for capping and proofreading
Biological Source:
Source Organism(s):
Expression System(s):
Method Details:
Experimental Method:
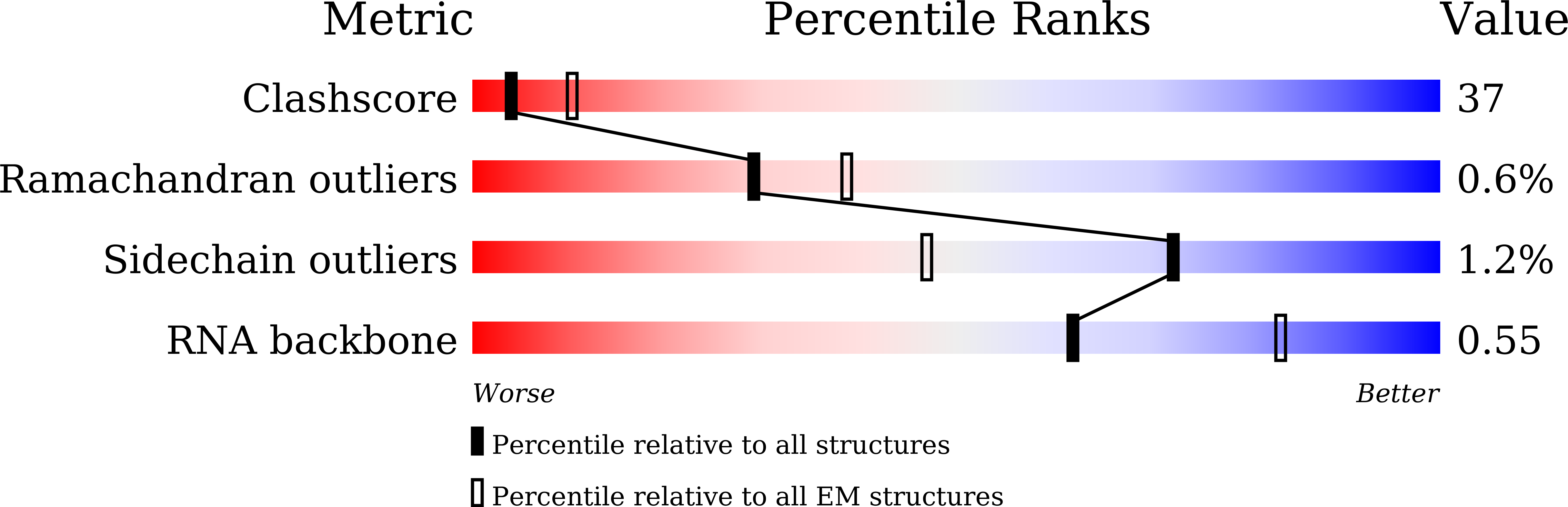
Resolution:
3.78 Å
Aggregation State:
PARTICLE
Reconstruction Method:
SINGLE PARTICLE