Deposition Date
2021-03-24
Release Date
2021-05-26
Last Version Date
2024-11-13
Entry Detail
PDB ID:
7EGK
Keywords:
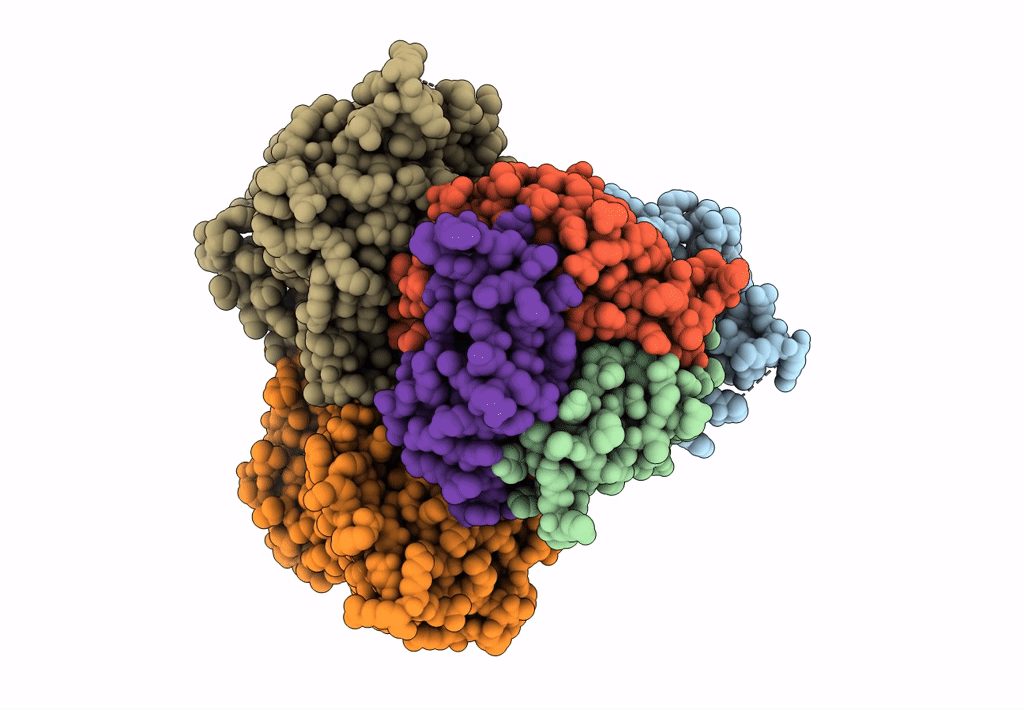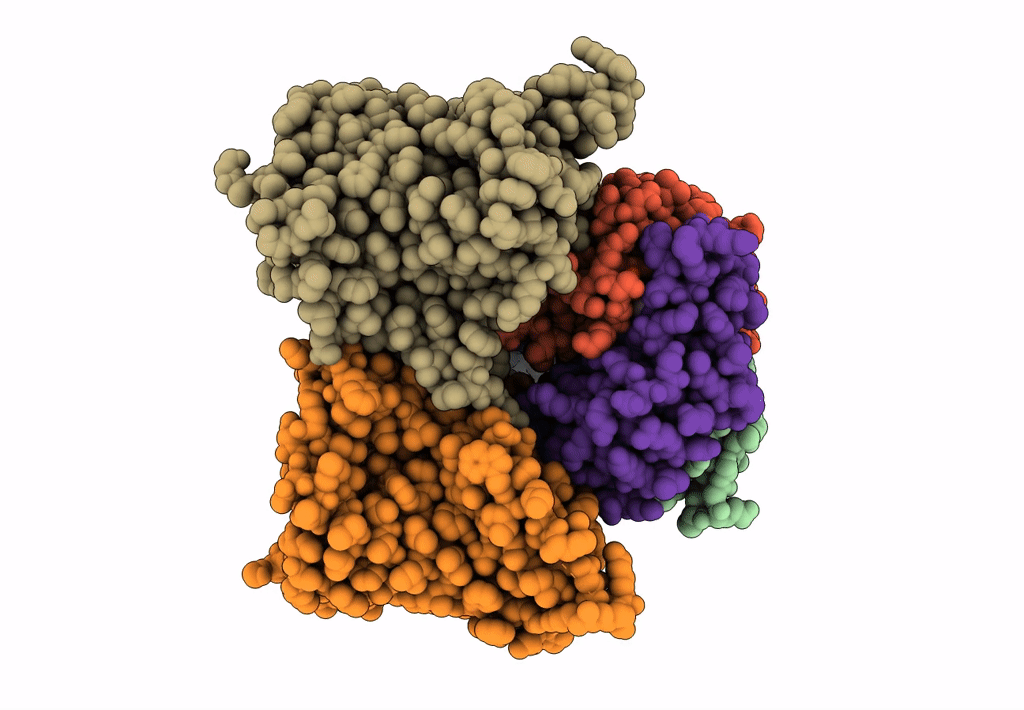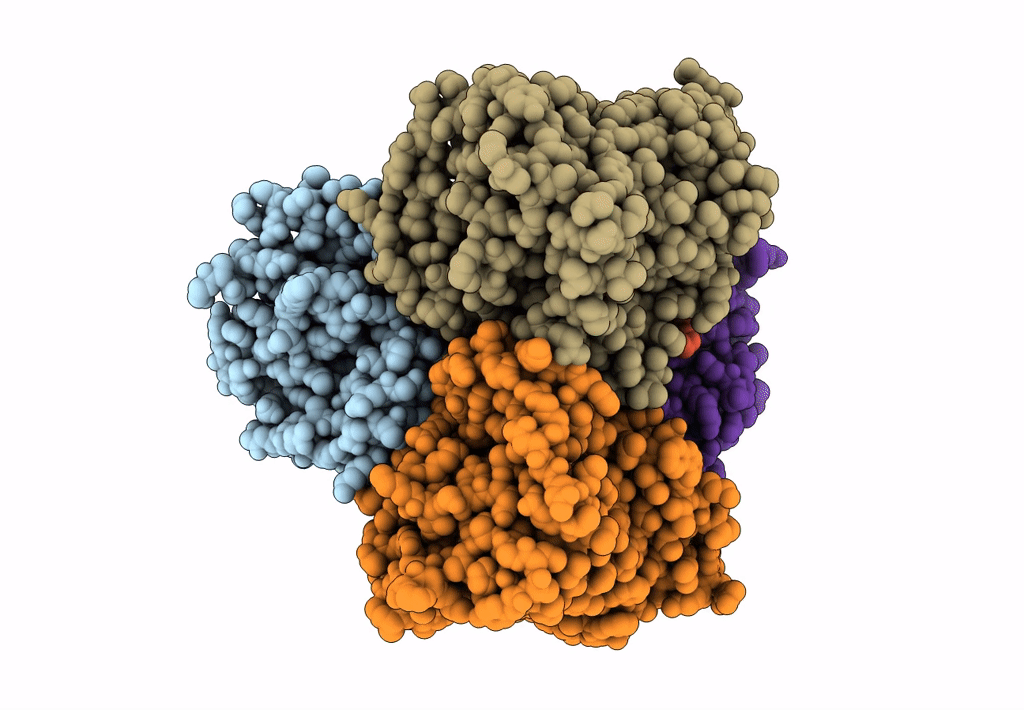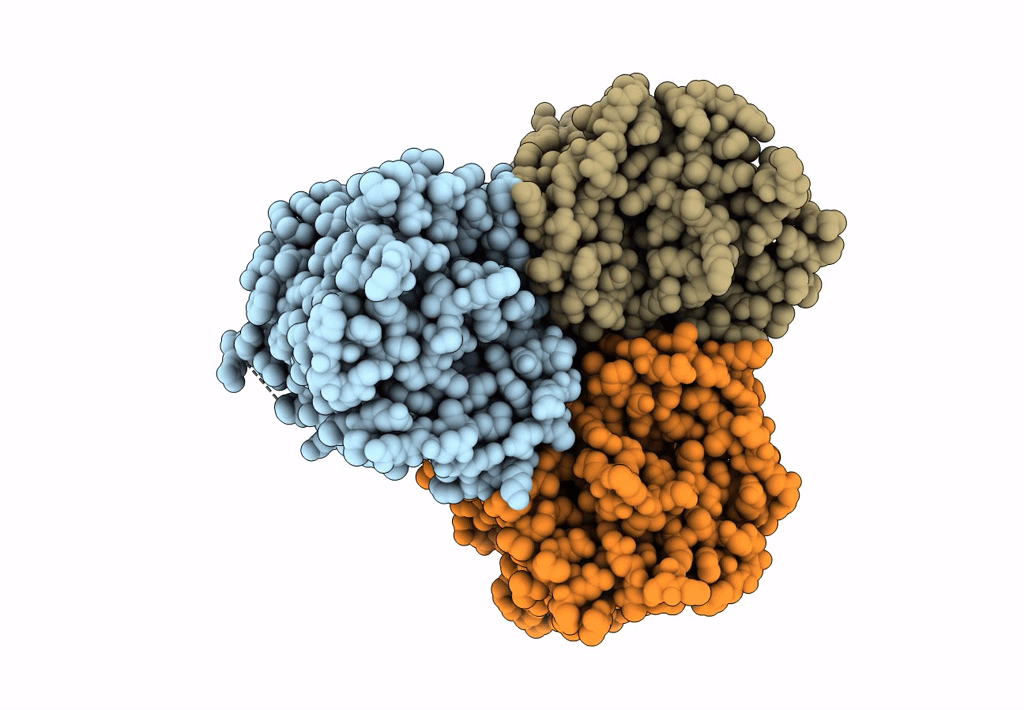
Title:
Bicarbonate transporter complex SbtA-SbtB bound to AMP
Biological Source:
Source Organism(s):
Synechocystis sp. (strain PCC 6803 / Kazusa) (Taxon ID: 1111708)
Expression System(s):
Method Details:
Experimental Method:
Resolution:
2.70 Å
Aggregation State:
PARTICLE
Reconstruction Method:
SINGLE PARTICLE


