Deposition Date
2020-10-26
Release Date
2021-03-17
Last Version Date
2024-03-27
Entry Detail
PDB ID:
7DCO
Keywords:
Title:
Cryo-EM structure of the activated spliceosome (Bact complex) at an atomic resolution of 2.5 angstrom
Biological Source:
Source Organism(s):
Saccharomyces cerevisiae (Taxon ID: 4932)
Saccharomyces cerevisiae (strain ATCC 204508 / S288c) (Taxon ID: 559292)
Saccharomyces cerevisiae (strain ATCC 204508 / S288c) (Taxon ID: 559292)
Method Details:
Experimental Method:
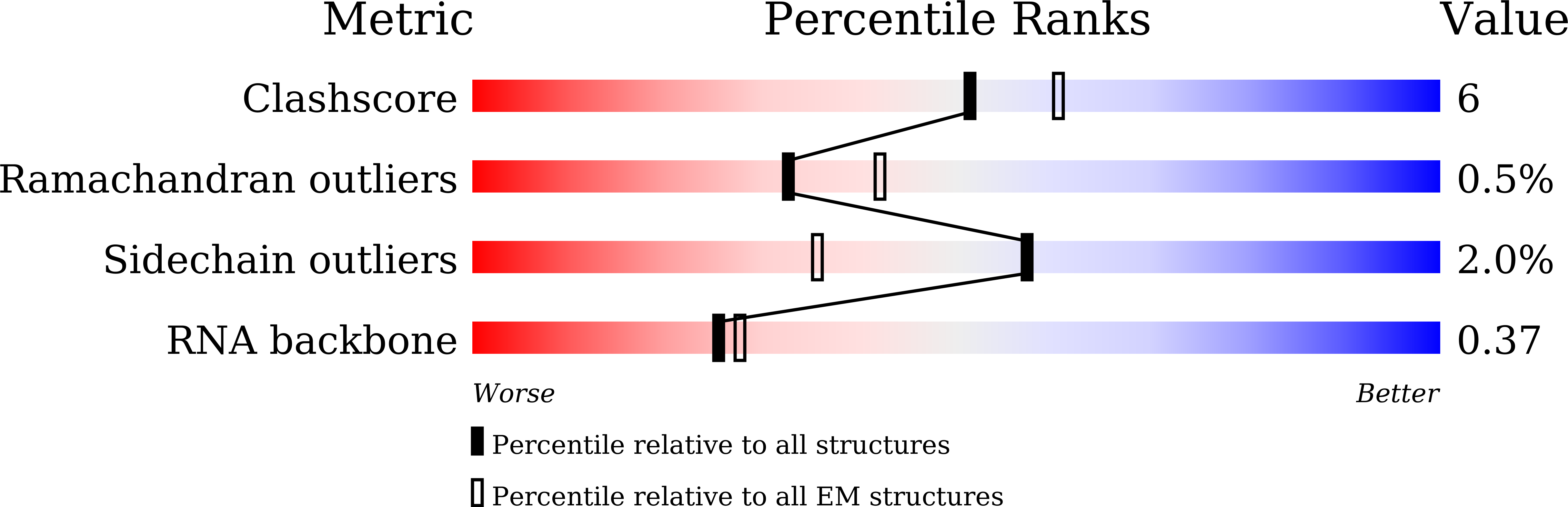
Resolution:
2.50 Å
Aggregation State:
PARTICLE
Reconstruction Method:
SINGLE PARTICLE