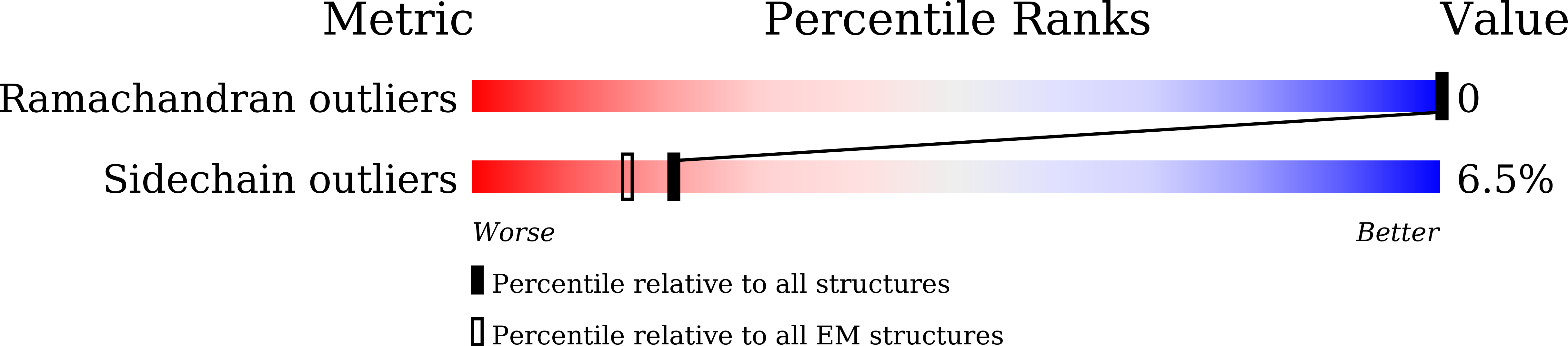Deposition Date
2020-10-07
Release Date
2021-05-19
Last Version Date
2024-03-27
Entry Detail
PDB ID:
7D84
Keywords:
Title:
34-fold symmetry Salmonella S ring formed by full-length FliF
Biological Source:
Source Organism(s):
Salmonella enterica serovar Typhimurium (Taxon ID: 90371)
Expression System(s):
Method Details:
Experimental Method:
Resolution:
3.70 Å
Aggregation State:
PARTICLE
Reconstruction Method:
SINGLE PARTICLE