Deposition Date
2020-09-27
Release Date
2021-06-09
Last Version Date
2024-11-20
Entry Detail
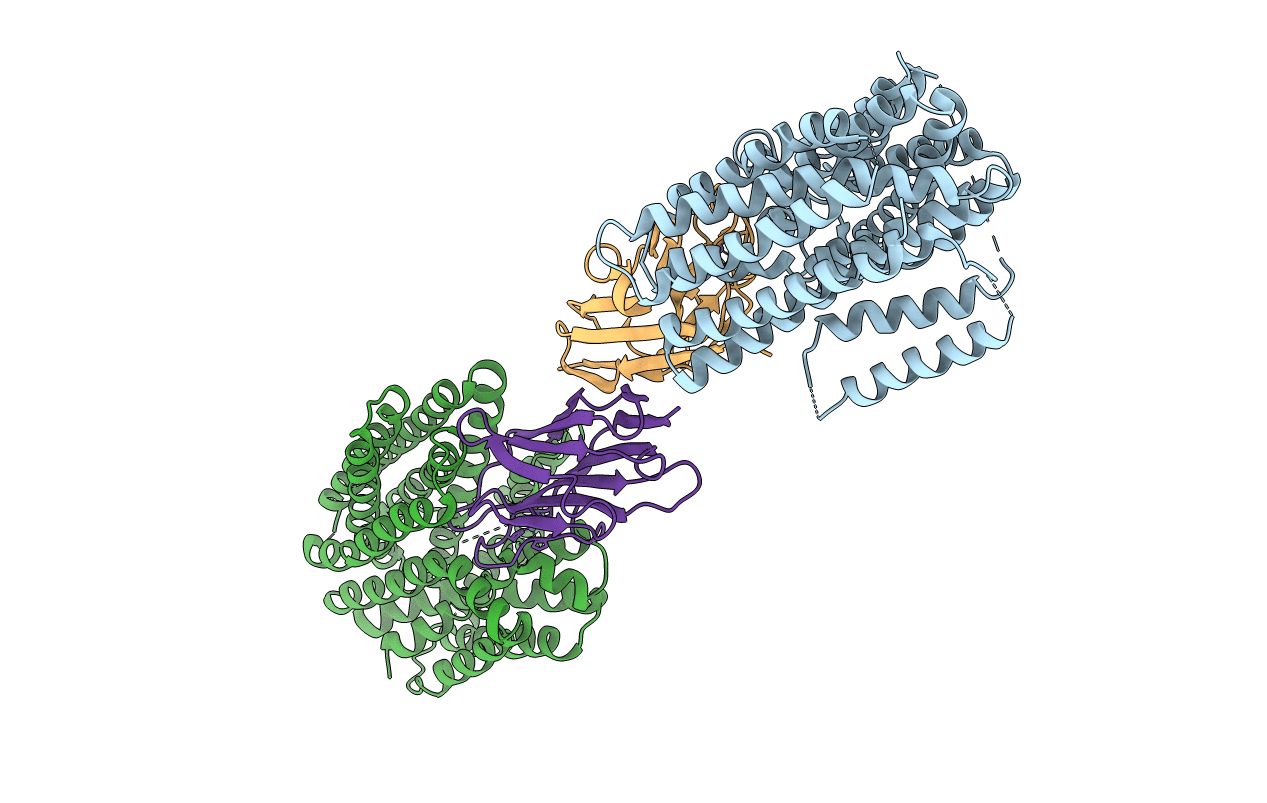
PDB ID:
7D5Q
Keywords:
Title:
Structure of NorC transporter (K398A mutant) in an outward-open conformation in complex with a single-chain Indian camelid antibody
Biological Source:
Source Organism(s):
Staphylococcus aureus subsp. aureus COL (Taxon ID: 93062)
Camelus dromedarius (Taxon ID: 9838)
Camelus dromedarius (Taxon ID: 9838)
Expression System(s):
Method Details:
Experimental Method:
Resolution:
3.60 Å
R-Value Free:
0.31
R-Value Work:
0.30
R-Value Observed:
0.30
Space Group:
P 1 21 1


