Deposition Date
2020-07-07
Release Date
2021-01-27
Last Version Date
2024-10-30
Entry Detail
PDB ID:
7CIG
Keywords:
Title:
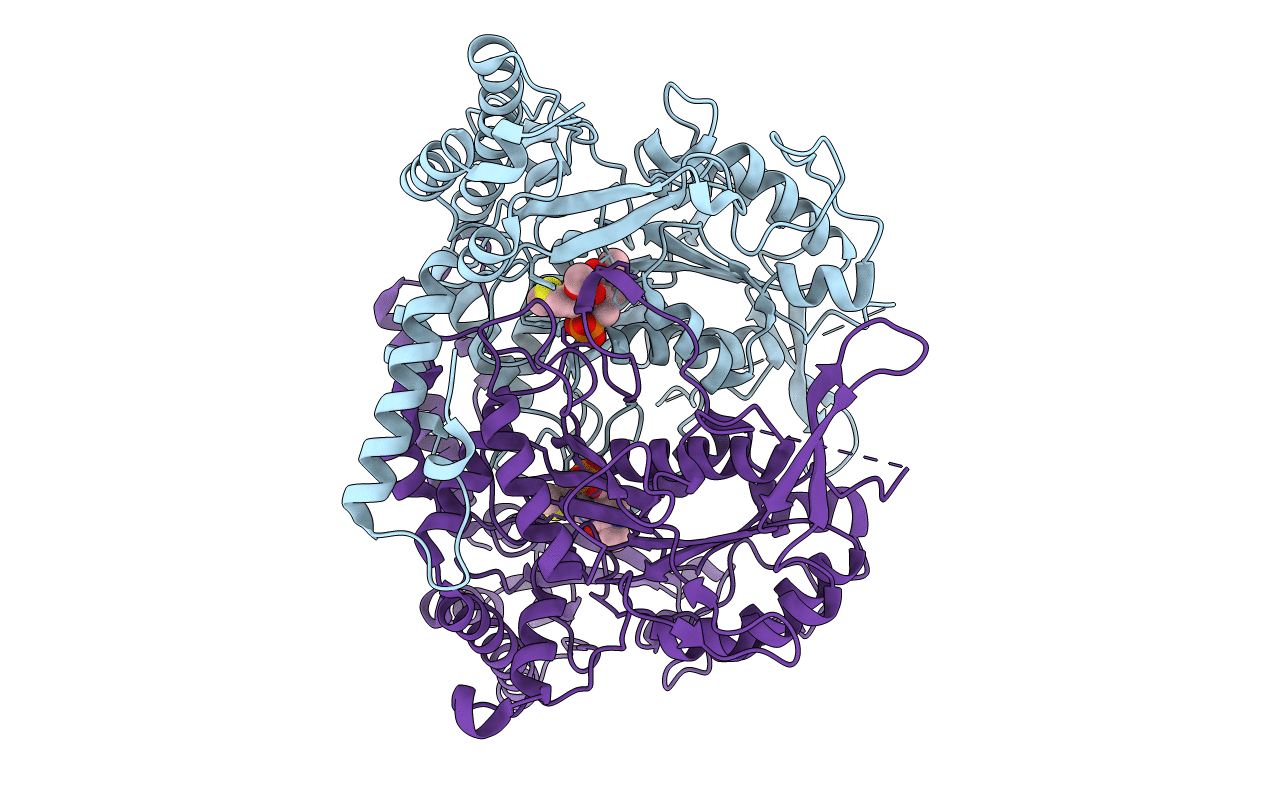
Crystal structure of L-methionine decarboxylase Q64A mutant from Streptomyces sp.590 in complexed with L- methionine methyl ester (geminal diamine form).
Biological Source:
Source Organism(s):
Streptomyces sp. 590 KI-2014 (Taxon ID: 1510823)
Expression System(s):
Method Details:
Experimental Method:
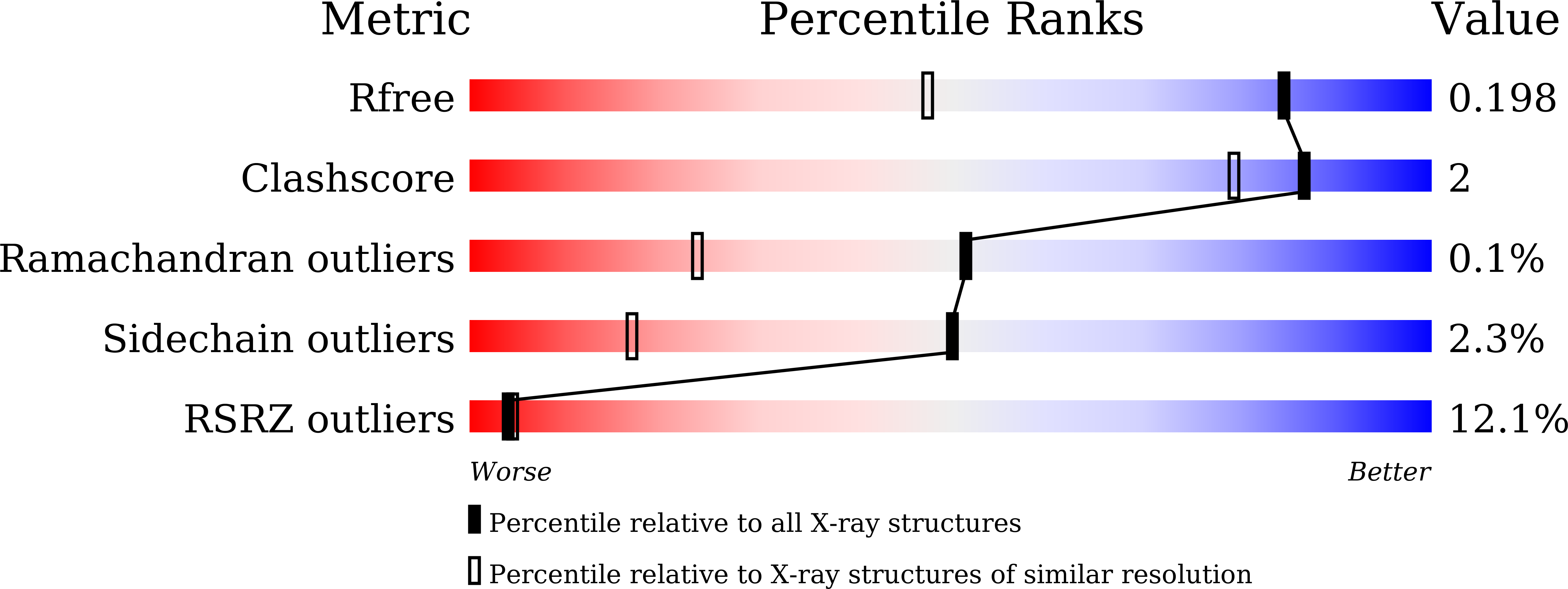
Resolution:
1.45 Å
R-Value Free:
0.19
R-Value Work:
0.17
R-Value Observed:
0.17
Space Group:
C 1 2 1