Deposition Date
2020-04-10
Release Date
2020-04-29
Last Version Date
2024-03-27
Entry Detail
PDB ID:
7BVG
Keywords:
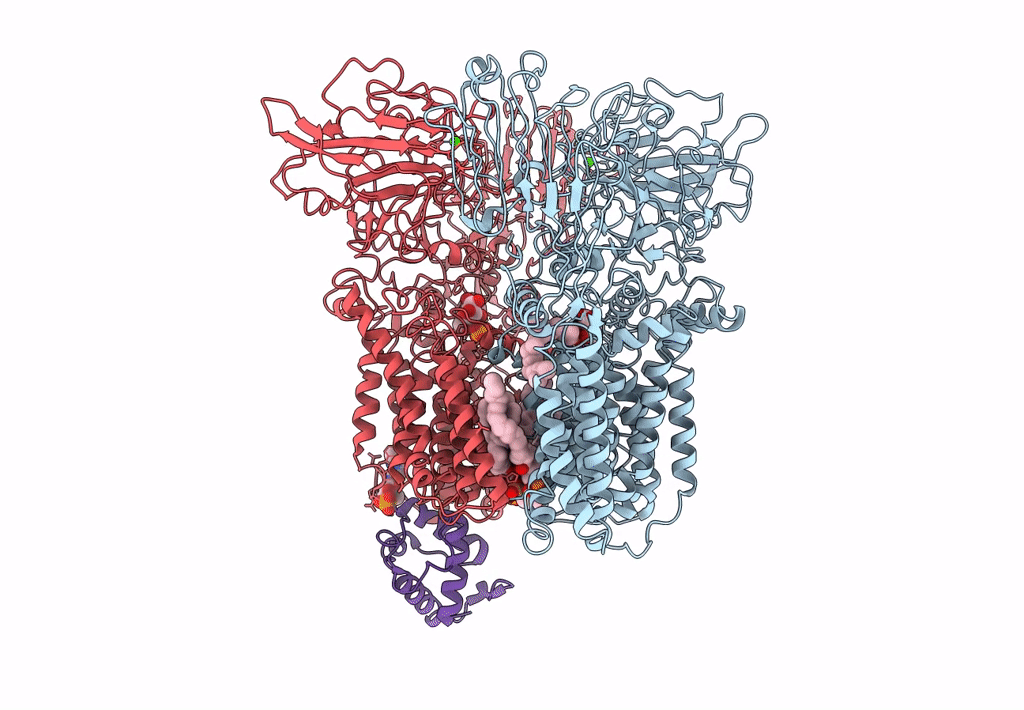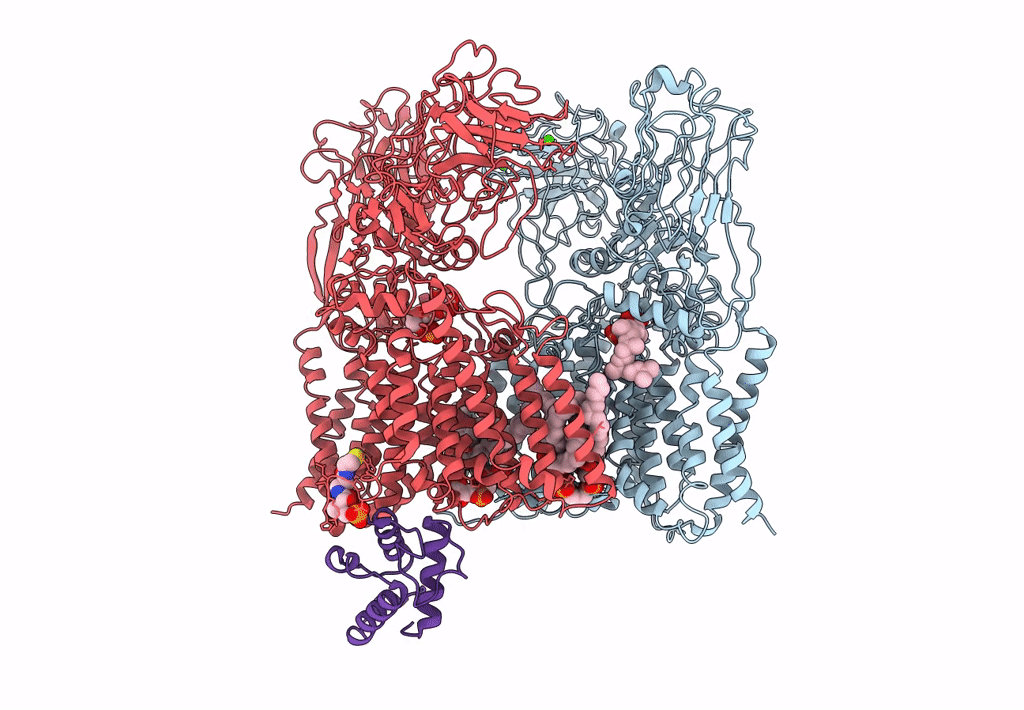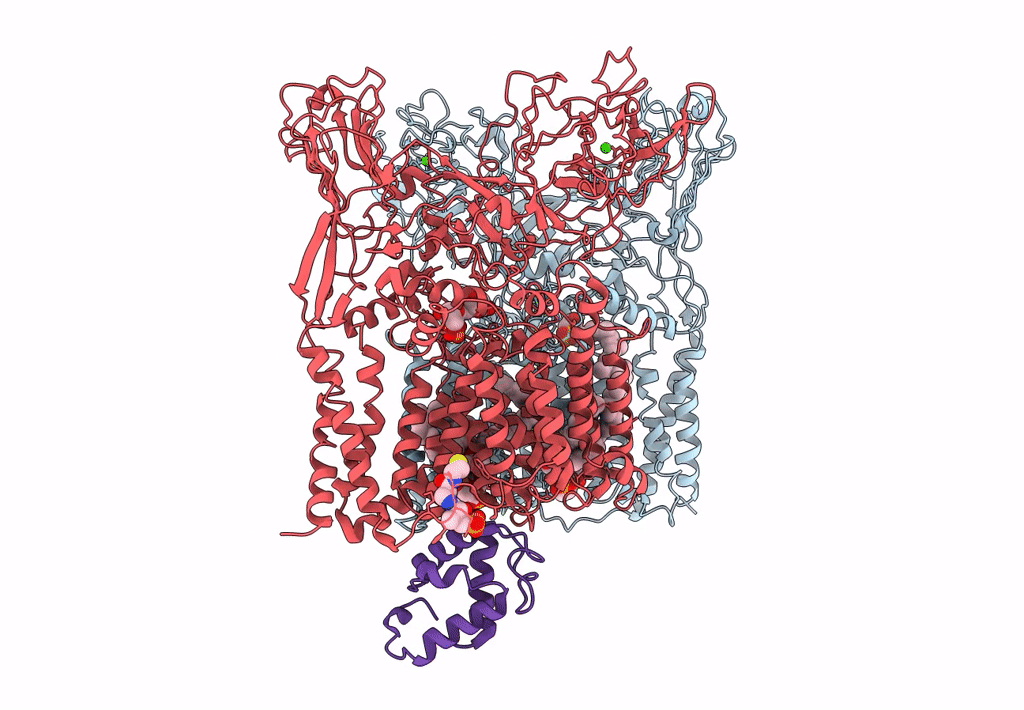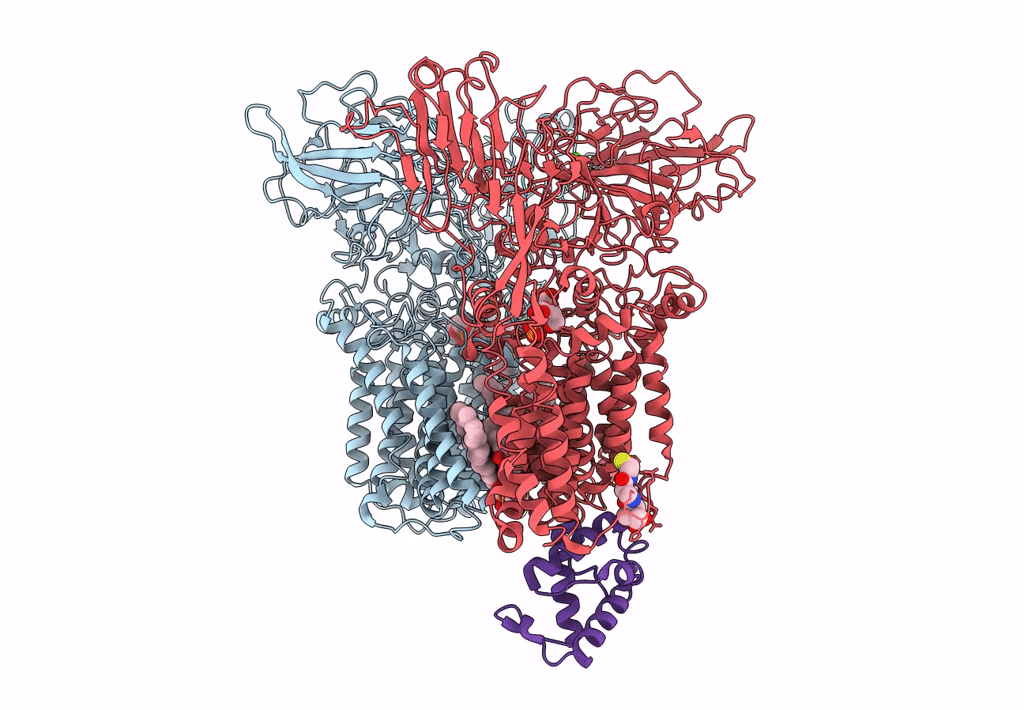
Title:
Cryo-EM structure of Mycobacterium smegmatis arabinosyltransferase EmbA-EmbB-AcpM2 in complex with di-arabinose.
Biological Source:
Source Organism:
Mycolicibacterium smegmatis MC2 155 (Taxon ID: 246196)
Host Organism:
Method Details:
Experimental Method:
Resolution:
3.10 Å
Aggregation State:
PARTICLE
Reconstruction Method:
SINGLE PARTICLE


