Deposition Date
2020-04-09
Release Date
2020-04-22
Last Version Date
2024-03-27
Entry Detail
PDB ID:
7BV2
Keywords:
Title:
The nsp12-nsp7-nsp8 complex bound to the template-primer RNA and triphosphate form of Remdesivir(RTP)
Biological Source:
Source Organism(s):
Expression System(s):
Method Details:
Experimental Method:
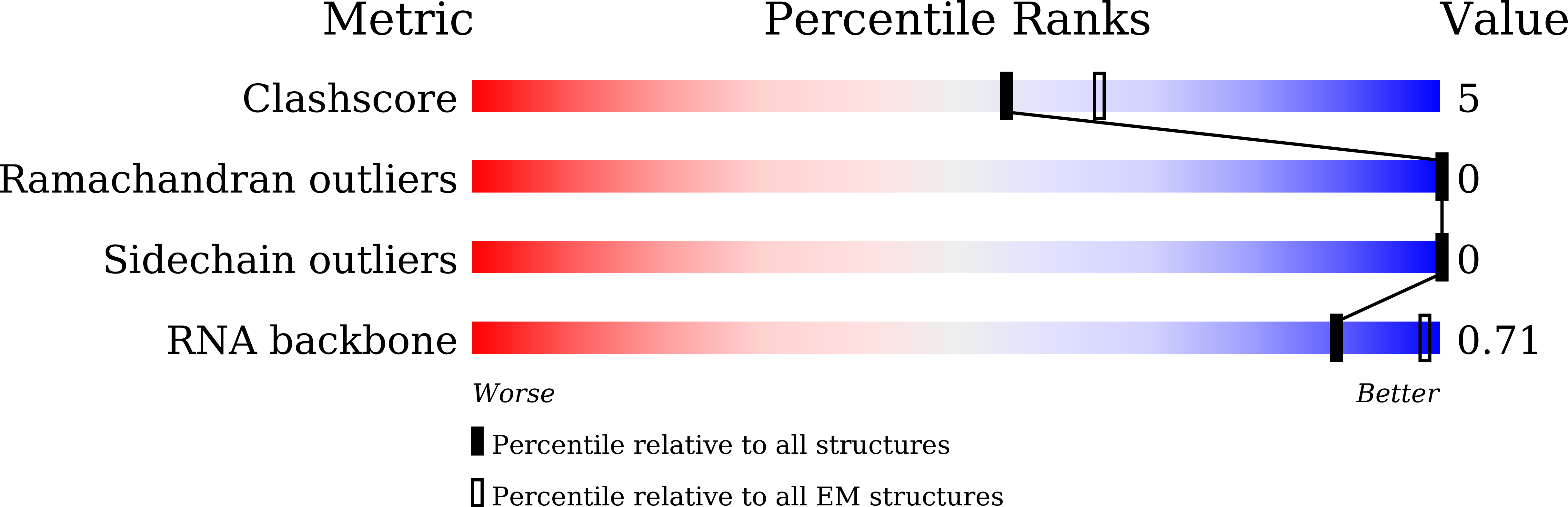
Resolution:
2.50 Å
Aggregation State:
PARTICLE
Reconstruction Method:
SINGLE PARTICLE