Deposition Date
2020-12-09
Release Date
2021-11-17
Last Version Date
2024-02-07
Entry Detail
PDB ID:
7B74
Keywords:
Title:
Chimeric Streptavidin With A Dimerization Domain For Artificial Transfer Hydrogenation
Biological Source:
Source Organism(s):
Streptomyces avidinii (Taxon ID: 1895)
Mycobacterium bovis (strain ATCC BAA-935 / AF2122/97) (Taxon ID: 233413)
Mycobacterium bovis (strain ATCC BAA-935 / AF2122/97) (Taxon ID: 233413)
Expression System(s):
Method Details:
Experimental Method:
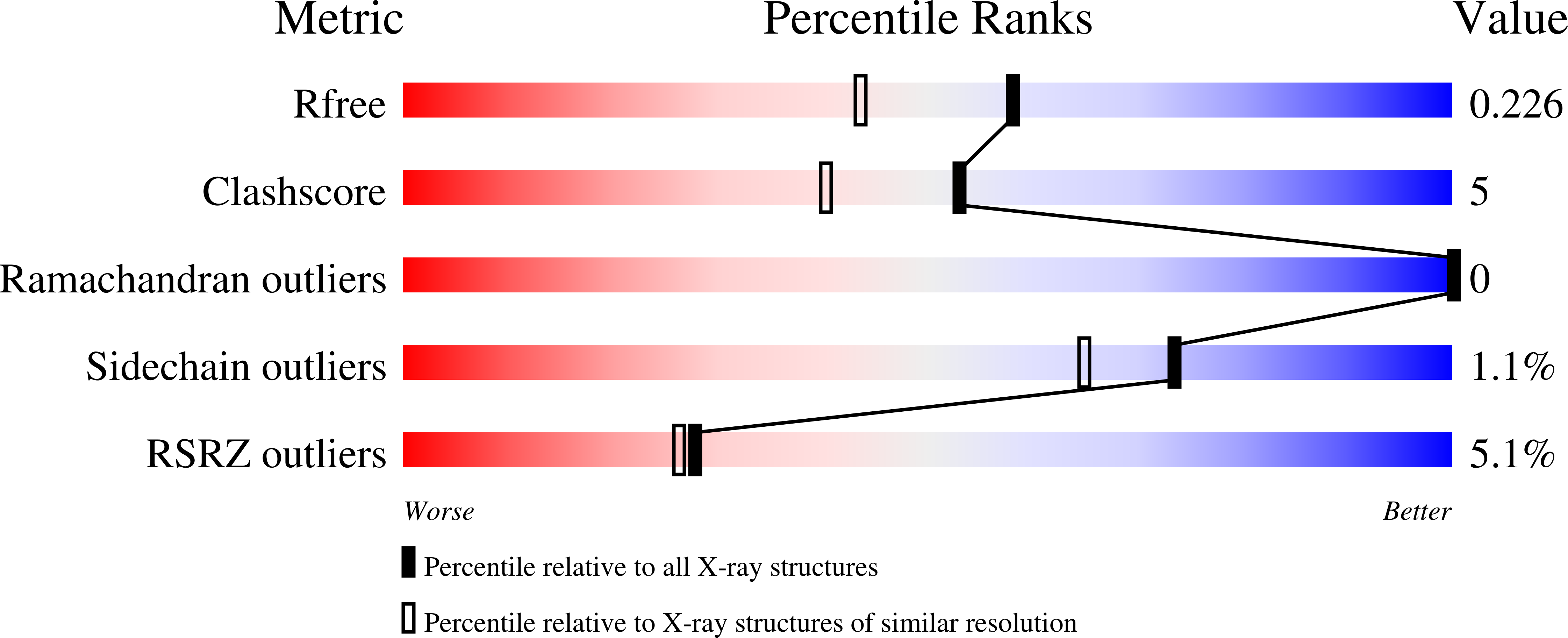
Resolution:
1.85 Å
R-Value Free:
0.21
R-Value Work:
0.19
Space Group:
P 1 21 1