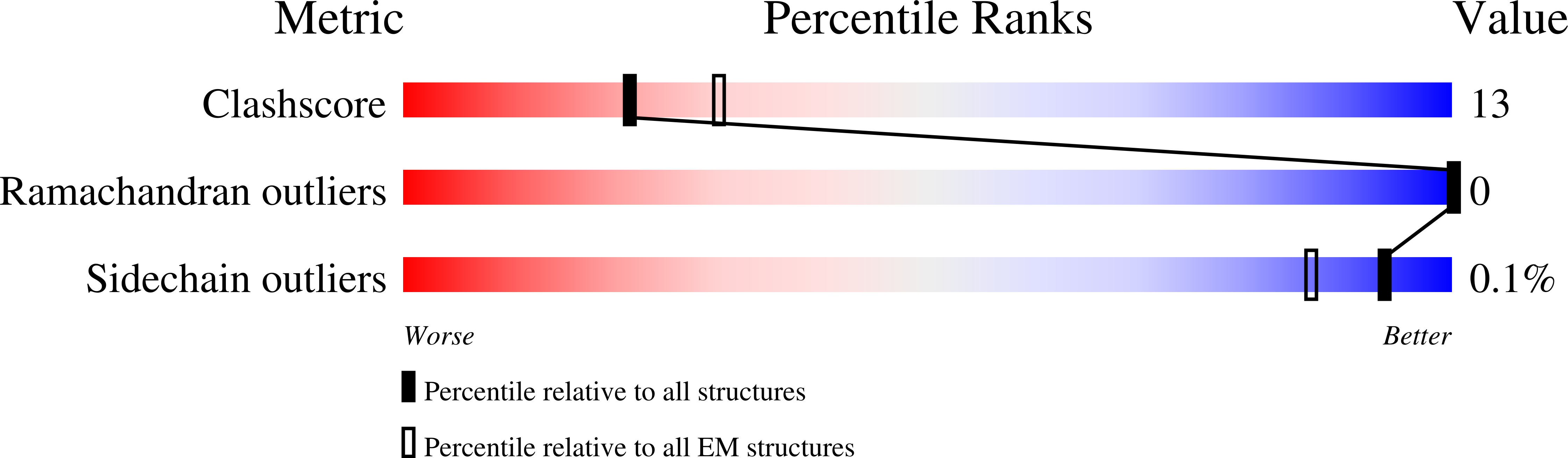
Deposition Date
2020-12-05
Release Date
2021-02-10
Last Version Date
2025-07-02
Entry Detail
PDB ID:
7B5N
Keywords:
Title:
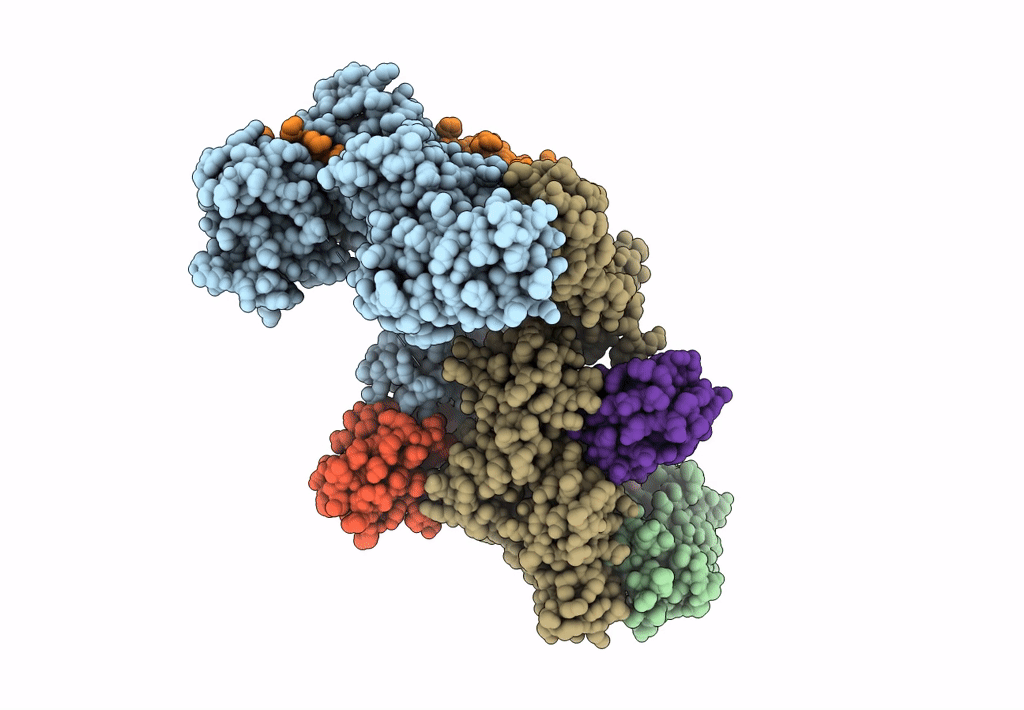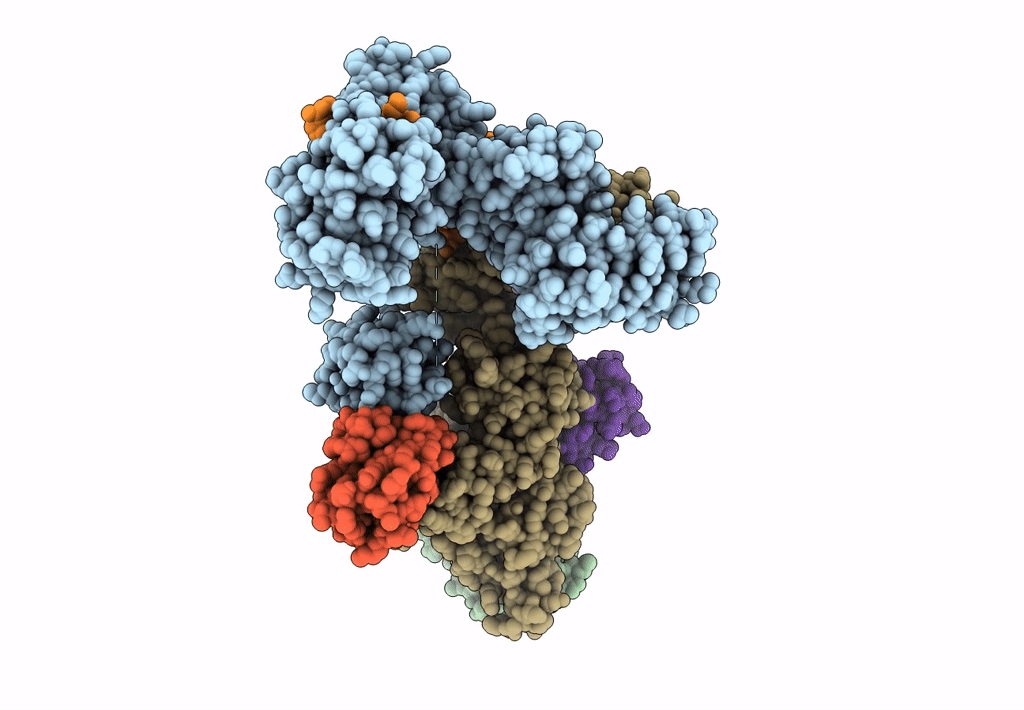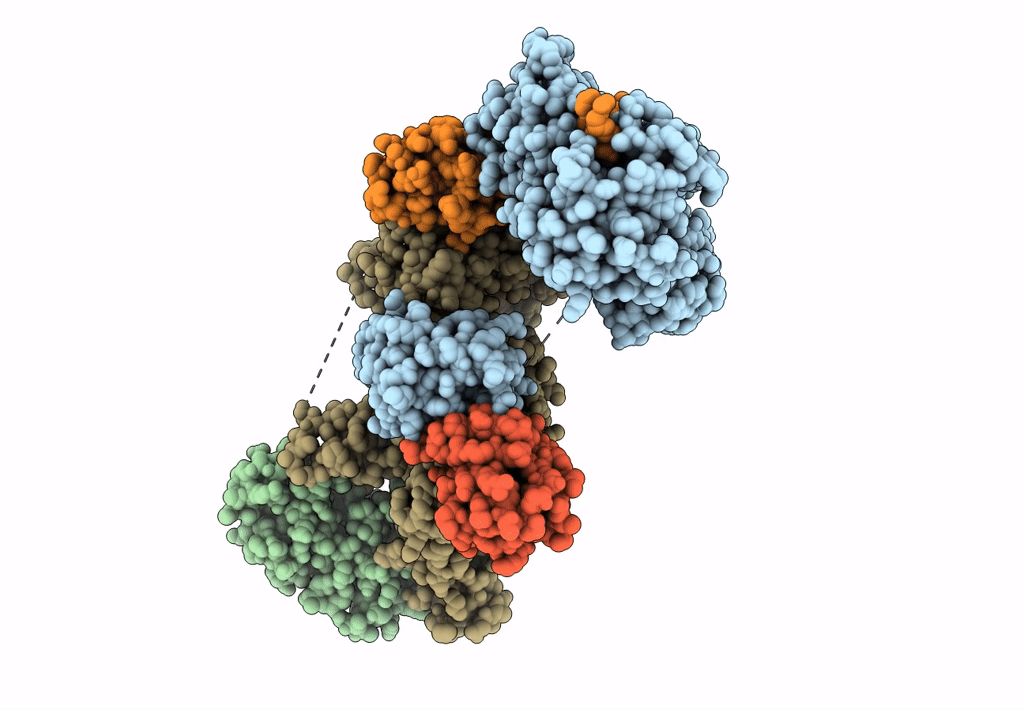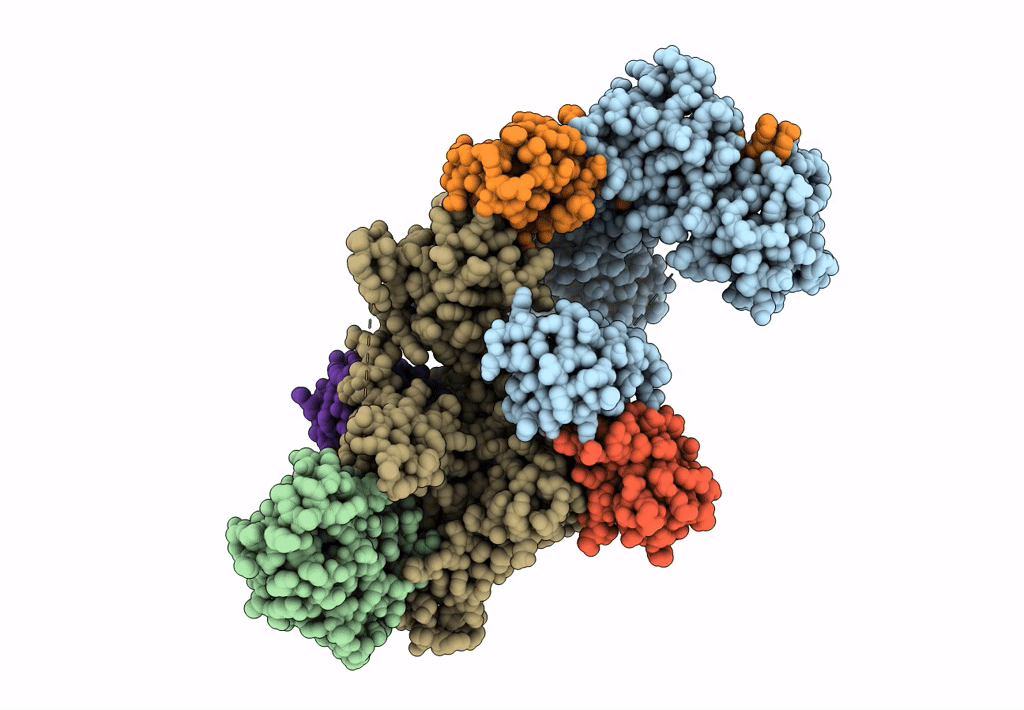
Ubiquitin ligation to F-box protein substrates by SCF-RBR E3-E3 super-assembly: NEDD8-CUL1-RBX1-UBE2L3~Ub~ARIH1.
Biological Source:
Source Organism(s):
Homo sapiens (Taxon ID: 9606)
Expression System(s):
Method Details:
Experimental Method:
Resolution:
3.60 Å
Aggregation State:
PARTICLE
Reconstruction Method:
SINGLE PARTICLE