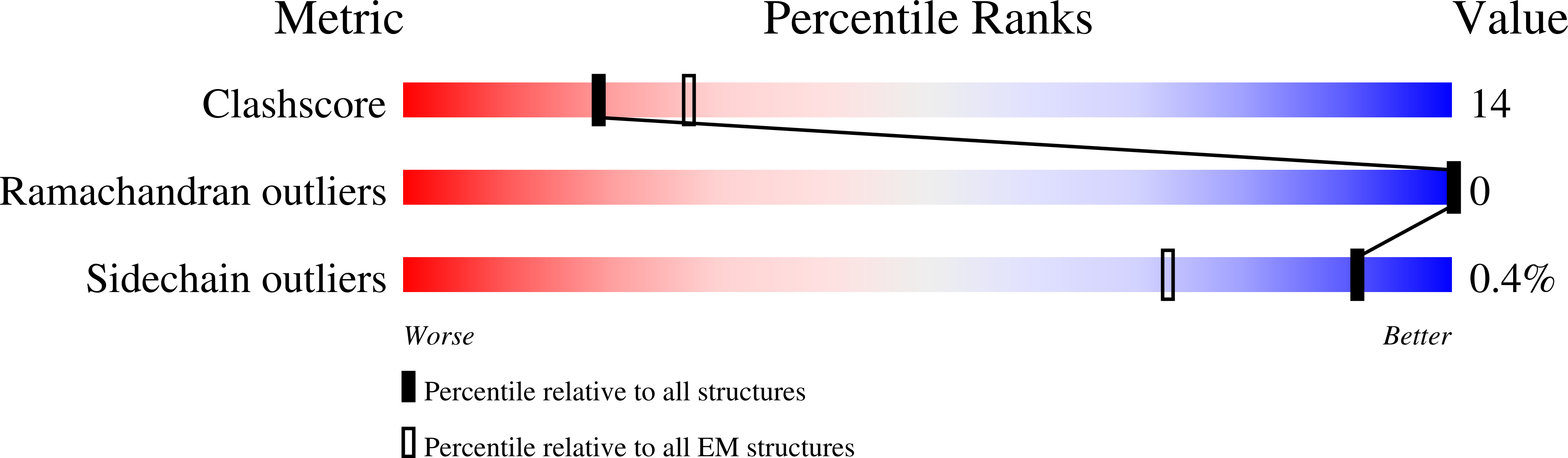Deposition Date
2020-12-05
Release Date
2021-02-17
Last Version Date
2025-07-09
Entry Detail
PDB ID:
7B5M
Keywords:
Title:
Ubiquitin ligation to F-box protein substrates by SCF-RBR E3-E3 super-assembly: CUL1-RBX1-SKP1-SKP2-CKSHS1-p27~Ub~ARIH1. Transition State 2
Biological Source:
Source Organism(s):
Homo sapiens (Taxon ID: 9606)
Expression System(s):
Method Details:
Experimental Method:
Resolution:
3.91 Å
Aggregation State:
PARTICLE
Reconstruction Method:
SINGLE PARTICLE