Deposition Date
2020-12-02
Release Date
2021-12-15
Last Version Date
2024-01-31
Entry Detail
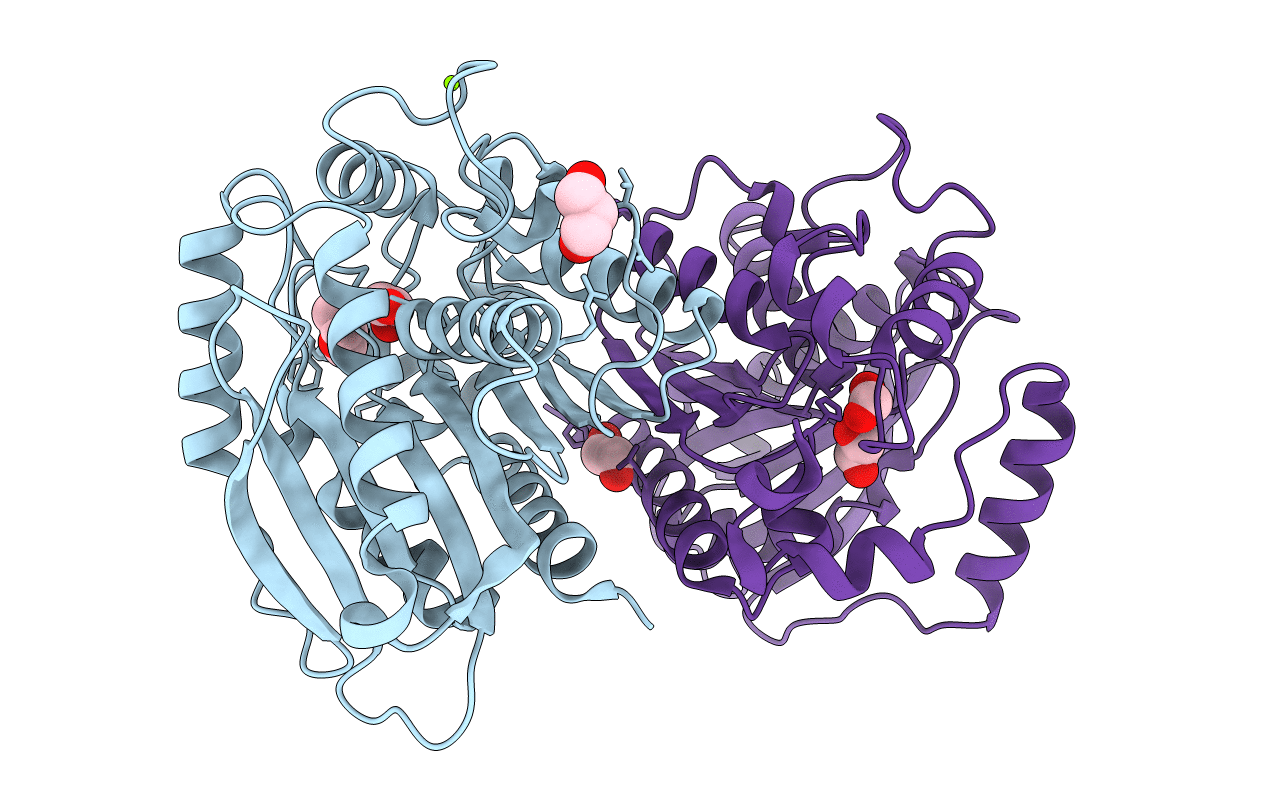
PDB ID:
7B4Q
Keywords:
Title:
Structure of a cold active HSL family esterase reveals mechanisms of low temperature adaptation and substrate specificity
Biological Source:
Source Organism(s):
Bacillus cohnii NBRC 15565 (Taxon ID: 1314751)
Expression System(s):
Method Details:
Experimental Method:
Resolution:
1.61 Å
R-Value Free:
0.19
R-Value Work:
0.16
R-Value Observed:
0.16
Space Group:
P 43 21 2


