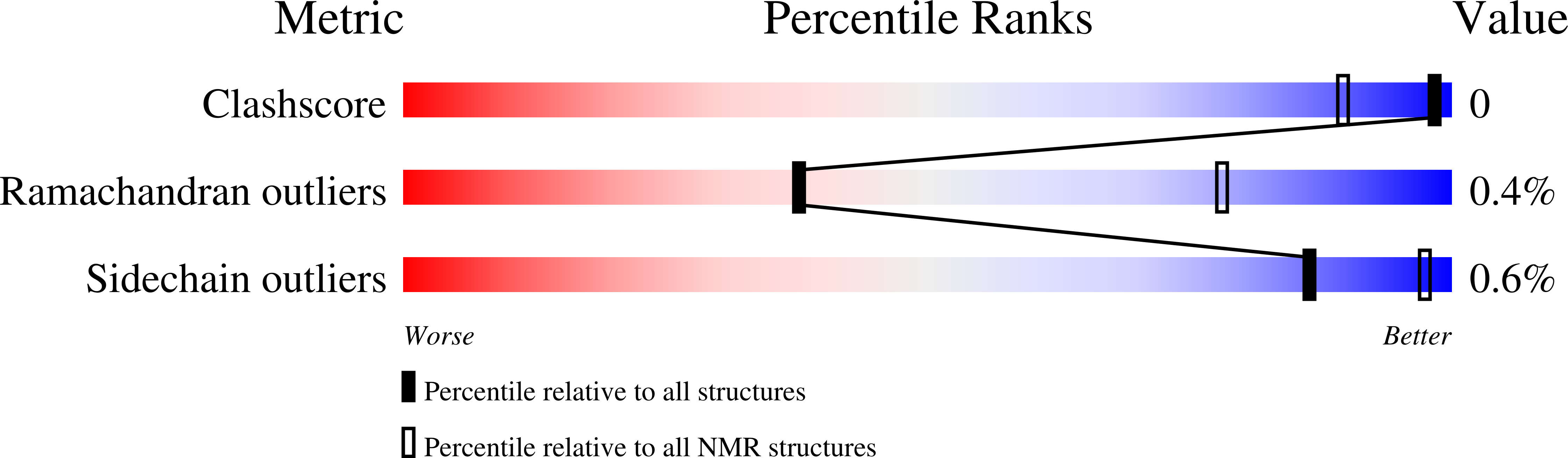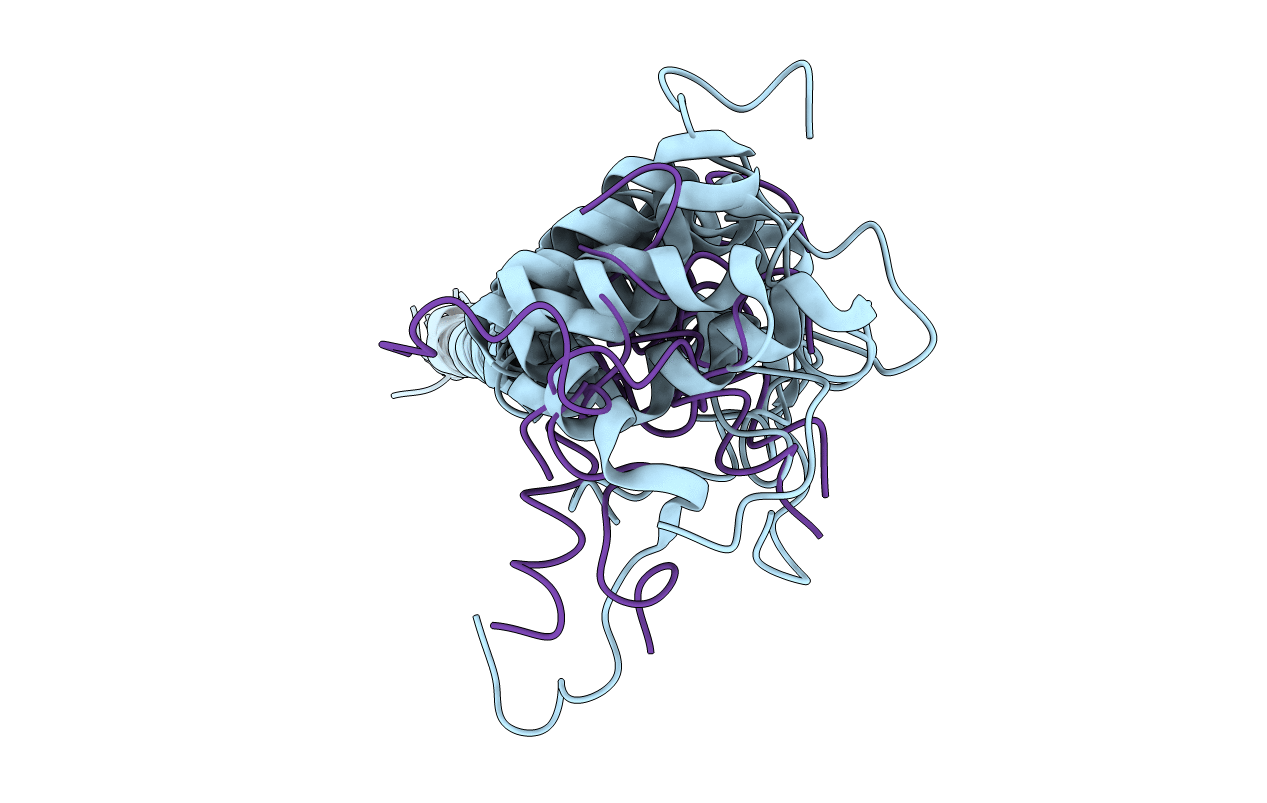
Deposition Date
2020-12-01
Release Date
2021-01-13
Last Version Date
2021-12-08
Entry Detail
PDB ID:
7B3J
Keywords:
Title:
Dynamic complex between all-D-enantiomeric peptide D3 with wild-type amyloid precursor protein 672-726 fragment (amyloid beta 1-55)
Biological Source:
Source Organism(s):
Homo sapiens (Taxon ID: 9606)
synthetic construct (Taxon ID: 32630)
synthetic construct (Taxon ID: 32630)
Expression System(s):
Method Details:
Experimental Method:
Conformers Calculated:
10
Conformers Submitted:
10
Selection Criteria:
structures with acceptable covalent geometry