Deposition Date
2020-11-19
Release Date
2020-12-16
Last Version Date
2024-11-13
Entry Detail
PDB ID:
7B0A
Keywords:
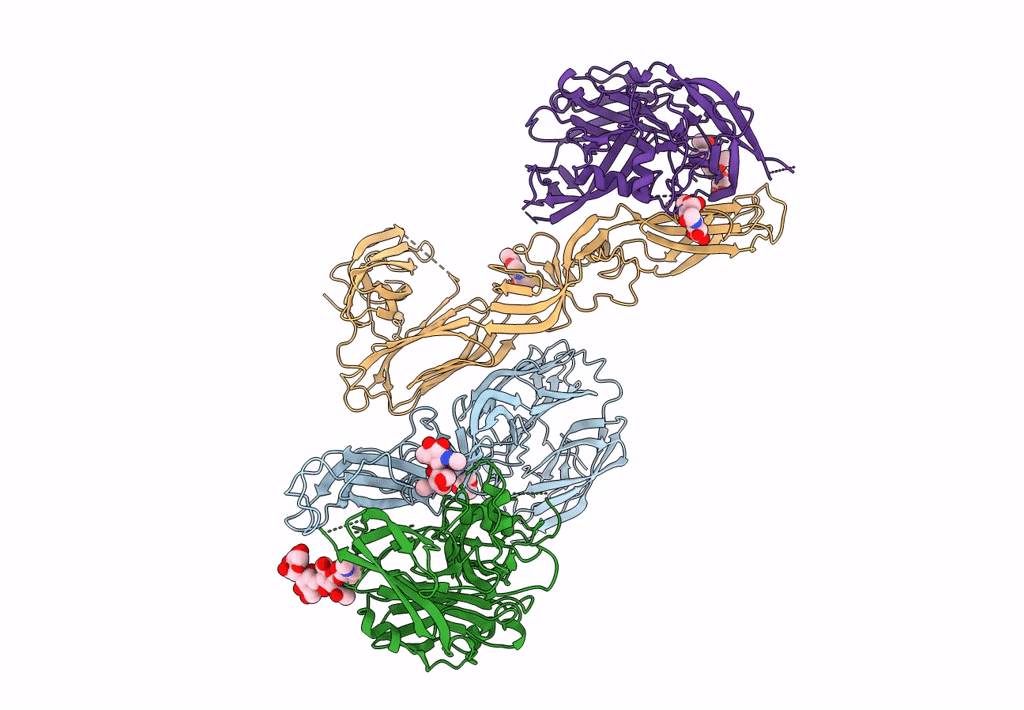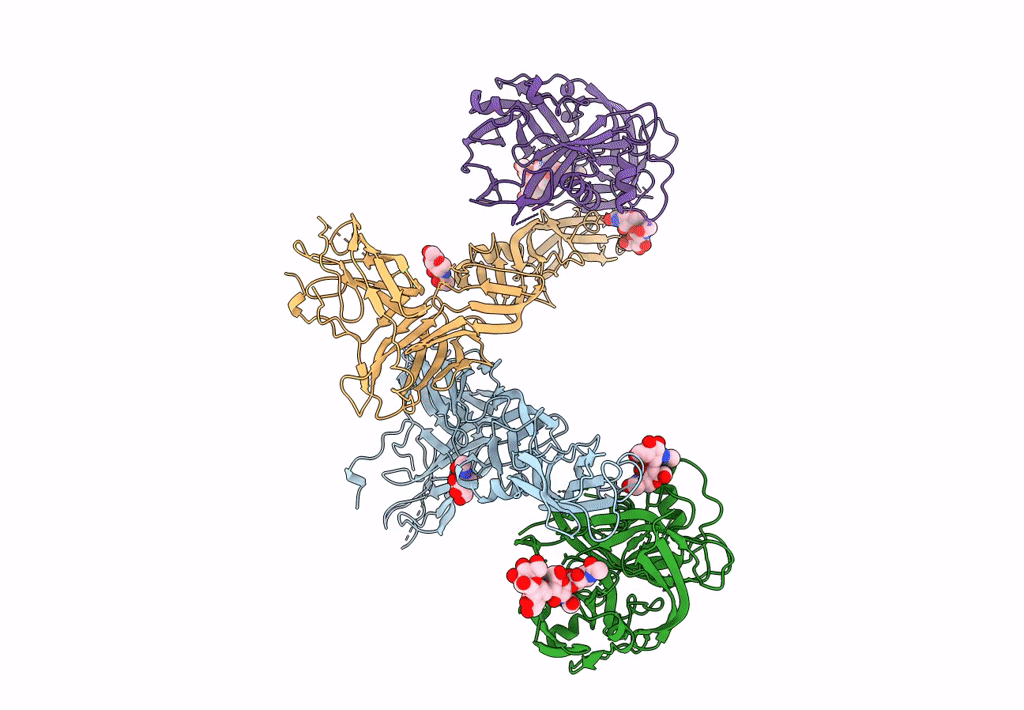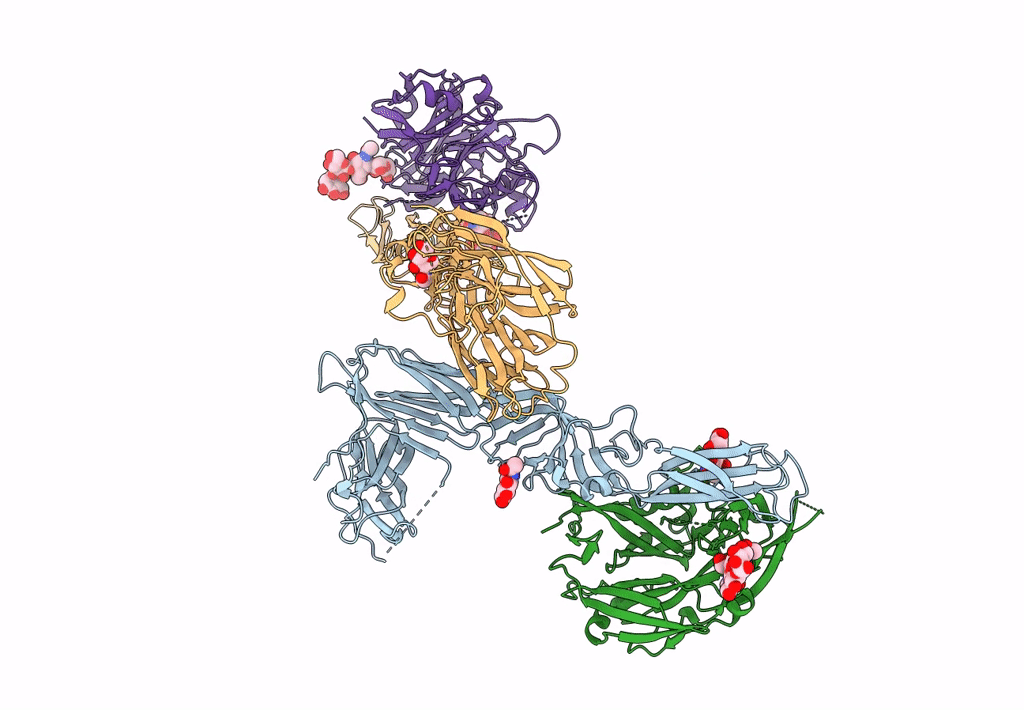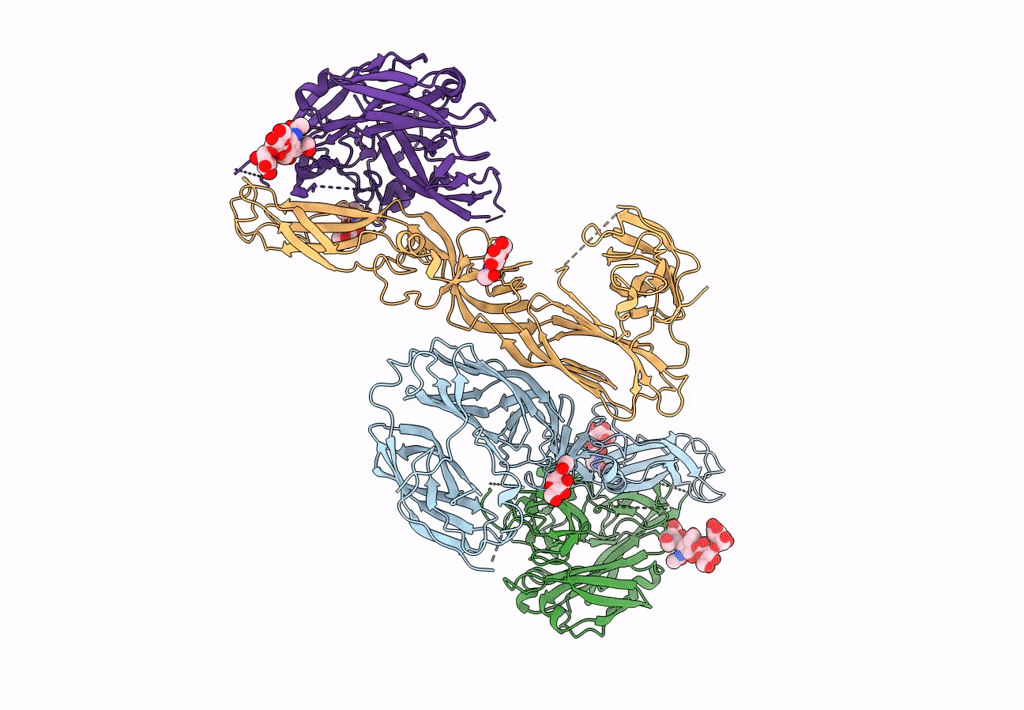
Title:
Puumala virus-like particle glycoprotein spike and lattice contacts model.
Biological Source:
Source Organism(s):
Puumala orthohantavirus (Taxon ID: 1980486)
Expression System(s):
Method Details:
Experimental Method:
Resolution:
13.90 Å
Aggregation State:
PARTICLE
Reconstruction Method:
SUBTOMOGRAM AVERAGING


