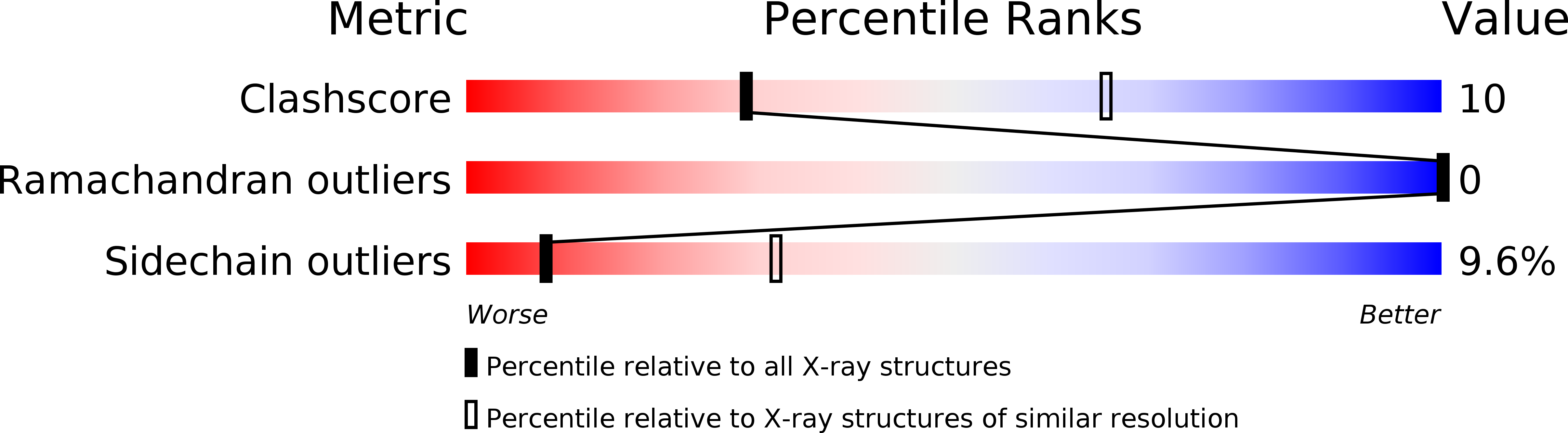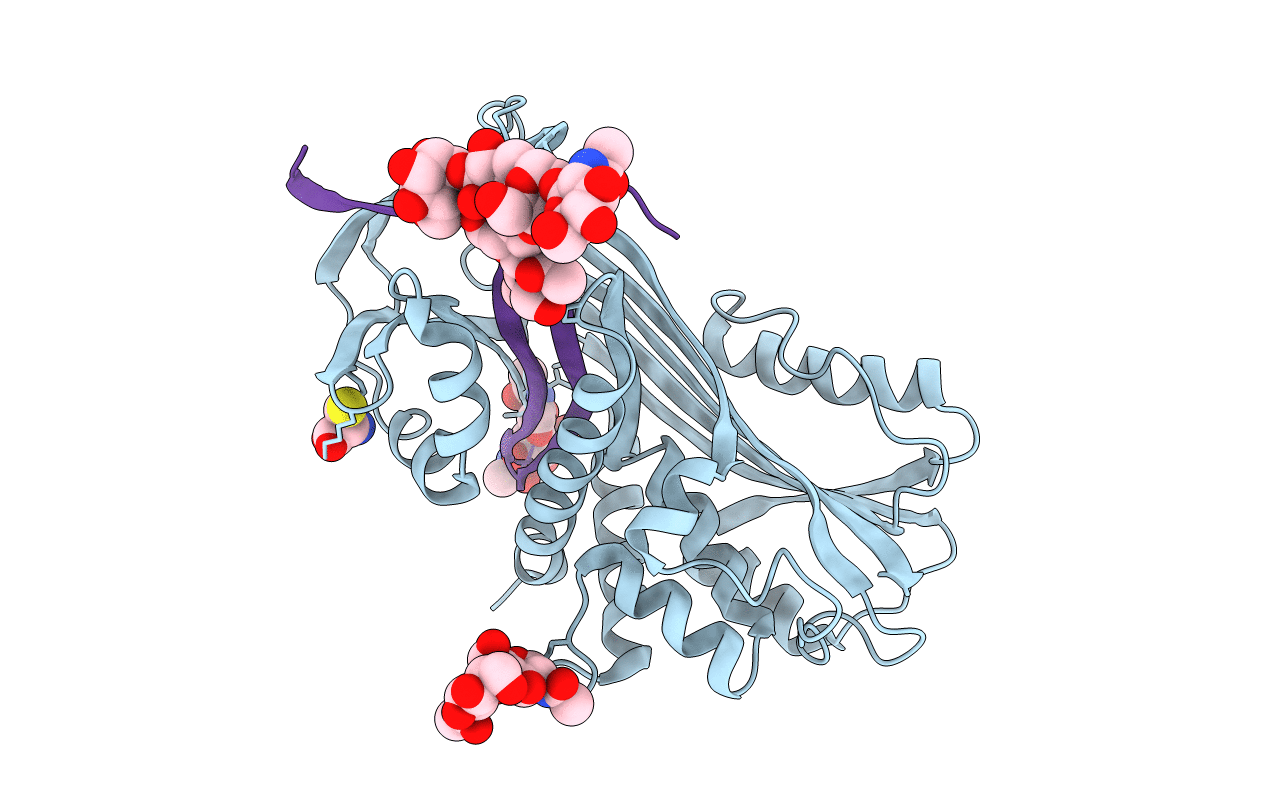
Deposition Date
1988-09-08
Release Date
1990-10-15
Last Version Date
2024-10-30
Entry Detail
PDB ID:
7API
Keywords:
Title:
THE S VARIANT OF HUMAN ALPHA1-ANTITRYPSIN, STRUCTURE AND IMPLICATIONS FOR FUNCTION AND METABOLISM
Biological Source:
Source Organism(s):
Homo sapiens (Taxon ID: 9606)
Method Details: