Deposition Date
2020-10-14
Release Date
2022-04-13
Last Version Date
2024-06-19
Entry Detail
PDB ID:
7AOJ
Keywords:
Title:
Plasmoredoxin, a redox-active protein unique for malaria parasites
Biological Source:
Source Organism:
Plasmodium falciparum (isolate 3D7) (Taxon ID: 36329)
Host Organism:
Method Details:
Experimental Method:
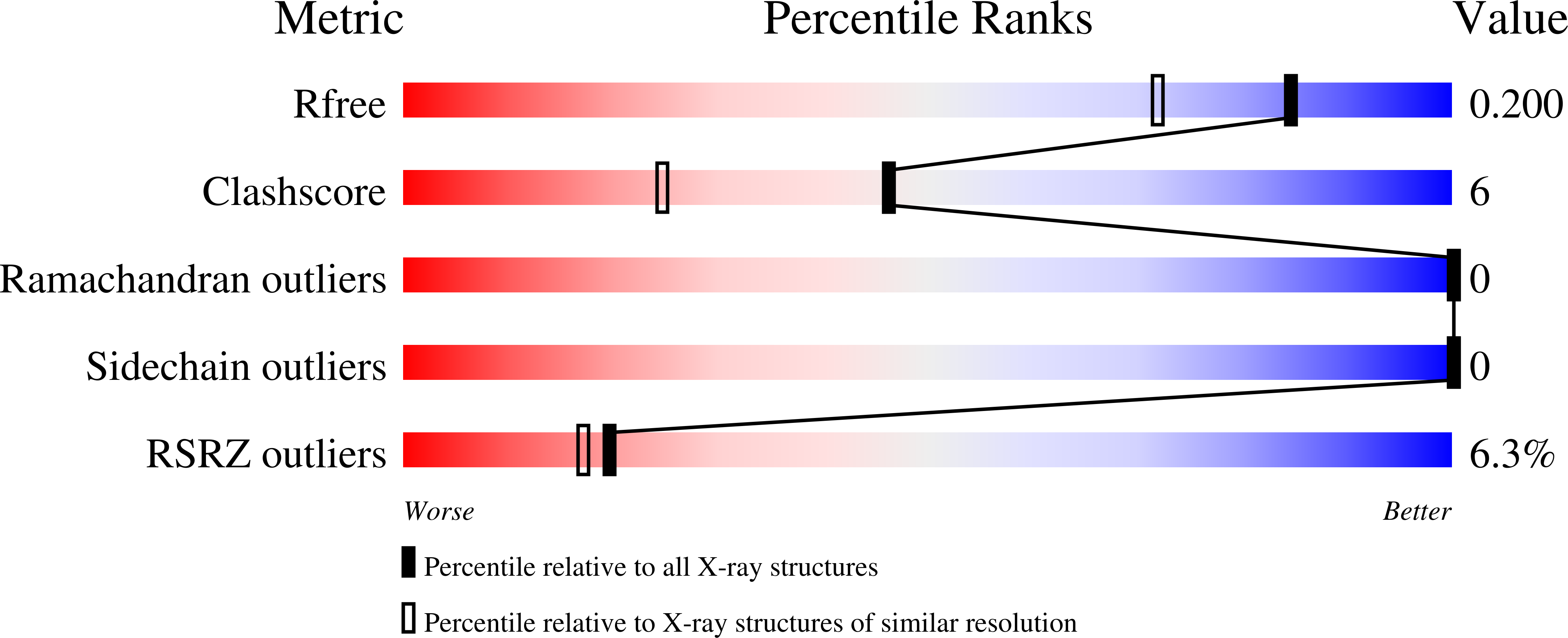
Resolution:
1.63 Å
R-Value Free:
0.19
R-Value Work:
0.16
R-Value Observed:
0.16
Space Group:
P 61