Abstact
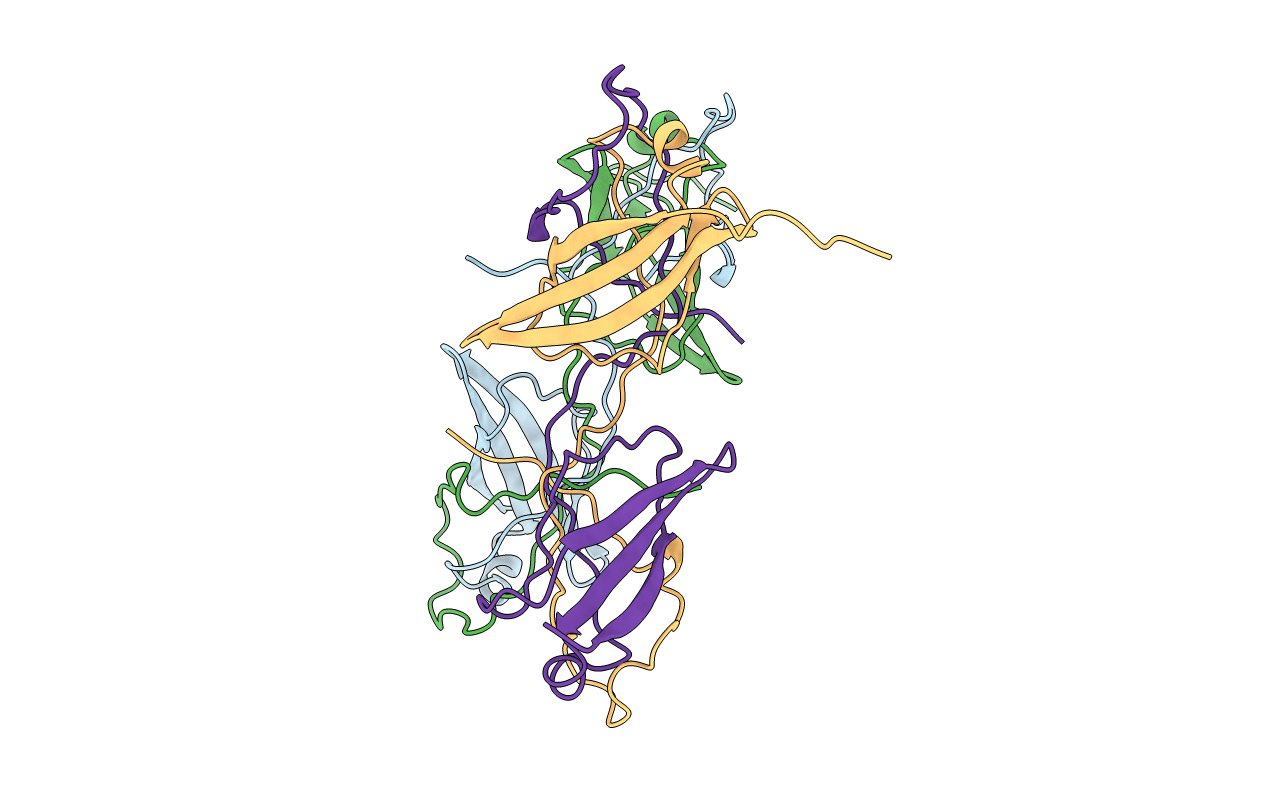
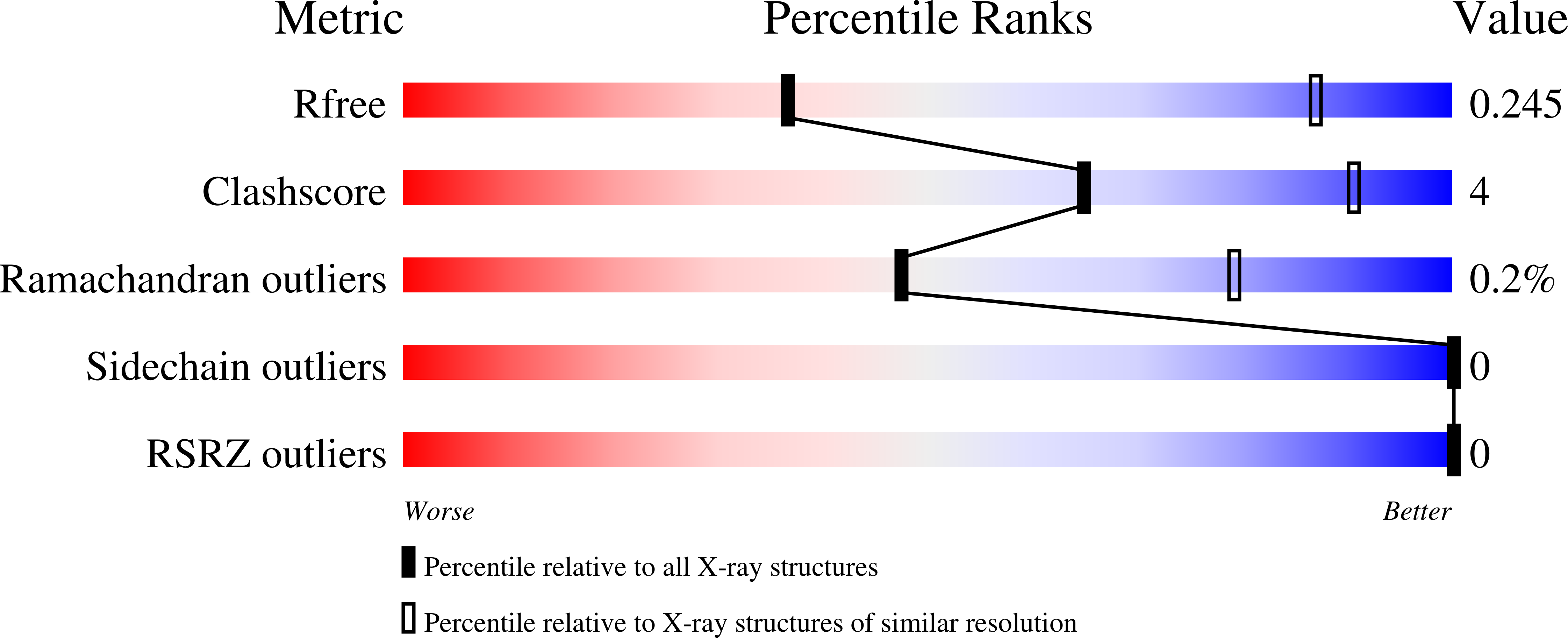
Mycobacterium tuberculosis (Mtb), which is responsible for more than a million deaths annually, uses lipids as the source of carbon and energy for its survival in the latent phase of infection. Mtb cannot synthesize all of the lipid molecules required for its growth and pathogenicity. Therefore, it relies on transporters such as the mammalian cell entry (Mce) complexes to import lipids from the host across the cell wall. Despite their importance for the survival and pathogenicity of Mtb, information on the structural properties of these proteins is not yet available. Each of the four Mce complexes in Mtb (Mce1-4) comprises six substrate-binding proteins (SBPs; MceA-F), each of which contains four conserved domains (N-terminal transmembrane, MCE, helical and C-terminal unstructured tail domains). Here, the properties of the various domains of Mtb Mce1A and Mce4A, which are involved in the import of mycolic/fatty acids and cholesterol, respectively, are reported. In the crystal structure of the MCE domain of Mce4A (MtMce4A39-140) a domain-swapped conformation is observed, whereas solution studies, including small-angle X-ray scattering (SAXS), indicate that all Mce1A and Mce4A domains are predominantly monomeric. Further, structural comparisons show interesting differences from the bacterial homologs MlaD, PqiB and LetB, which form homohexamers when assembled as functional transporter complexes. These data, and the fact that there are six SBPs in each Mtb mce operon, suggest that the MceA-F SBPs from Mce1-4 may form heterohexamers. Also, interestingly, the purification and SAXS analysis showed that the helical domains interact with the detergent micelle, suggesting that when assembled the helical domains of MceA-F may form a hydrophobic pore for lipid transport, as observed in EcPqiB. Overall, these data highlight the unique structural properties of the Mtb Mce SBPs.