Deposition Date
2020-09-01
Release Date
2020-11-04
Last Version Date
2024-01-31
Entry Detail
PDB ID:
7A99
Keywords:
Title:
Crystal structure of the Phe57Trp mutant of the arginine-bound form of domain 1 from TmArgBP
Biological Source:
Source Organism(s):
Thermotoga maritima (Taxon ID: 2336)
Thermotoga maritima (strain ATCC 43589 / MSB8 / DSM 3109 / JCM 10099) (Taxon ID: 243274)
Thermotoga maritima (strain ATCC 43589 / MSB8 / DSM 3109 / JCM 10099) (Taxon ID: 243274)
Expression System(s):
Method Details:
Experimental Method:
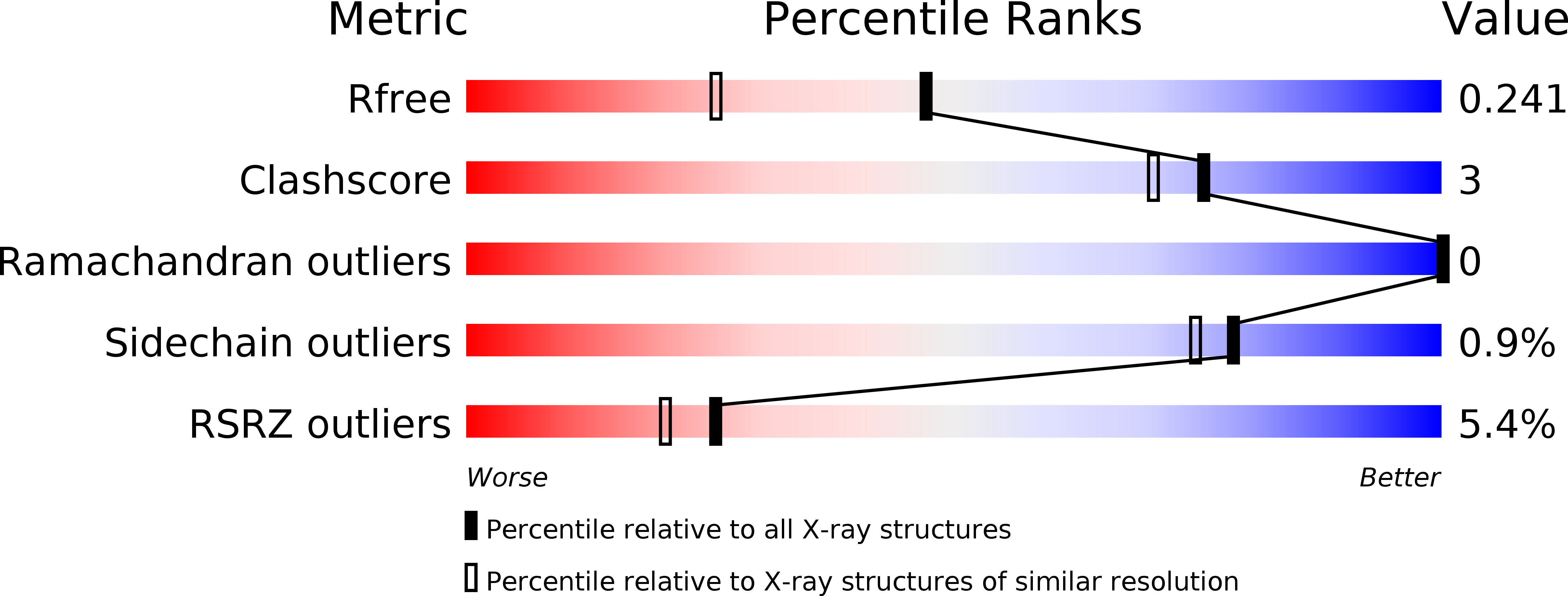
Resolution:
1.79 Å
R-Value Free:
0.23
R-Value Work:
0.17
R-Value Observed:
0.18
Space Group:
P 21 21 21