Deposition Date
2020-08-25
Release Date
2021-03-03
Last Version Date
2024-11-20
Entry Detail
PDB ID:
7A6O
Keywords:
Title:
Crystal Structure of the Complex of the Recombinant Von Willebrand Factor AIM-A1 domain and VHH81 at 2.1 Angstrom resolution
Biological Source:
Source Organism:
Homo sapiens (Taxon ID: 9606)
Lama glama (Taxon ID: 9844)
Lama glama (Taxon ID: 9844)
Host Organism:
Method Details:
Experimental Method:
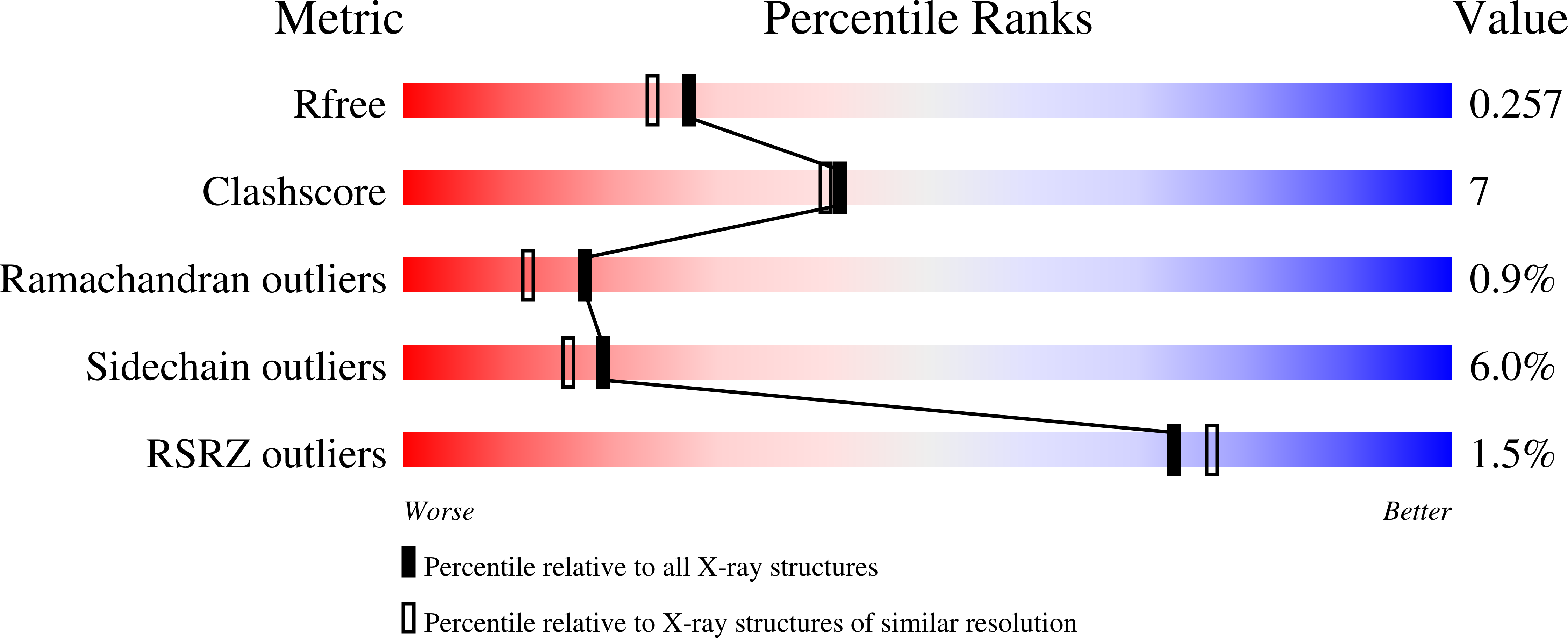
Resolution:
2.12 Å
R-Value Free:
0.25
R-Value Work:
0.19
Space Group:
P 43 21 2