Deposition Date
2020-08-10
Release Date
2021-08-18
Last Version Date
2024-01-31
Entry Detail
PDB ID:
7A0S
Keywords:
Title:
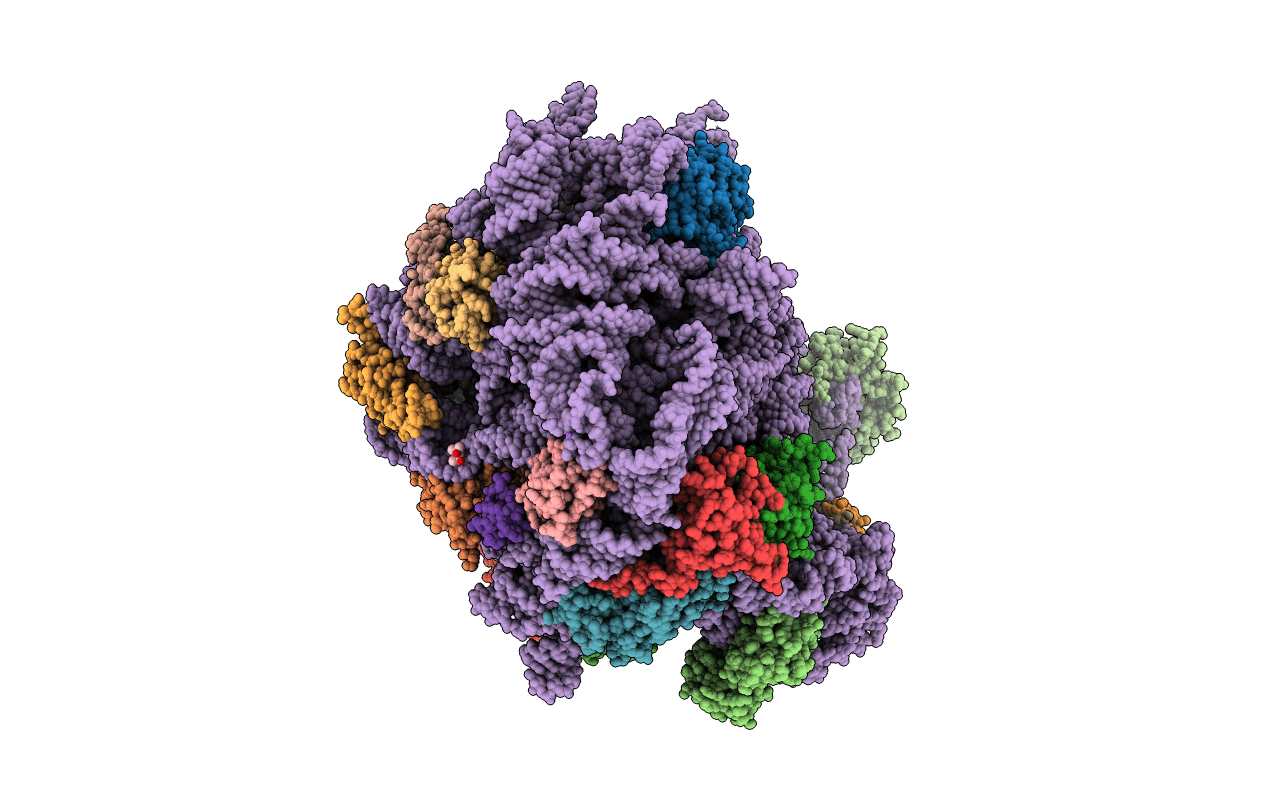
50S Deinococcus radiodurans ribosome bounded with mycinamicin I
Biological Source:
Source Organism(s):
Deinococcus radiodurans R1 (Taxon ID: 243230)
Method Details:
Experimental Method:
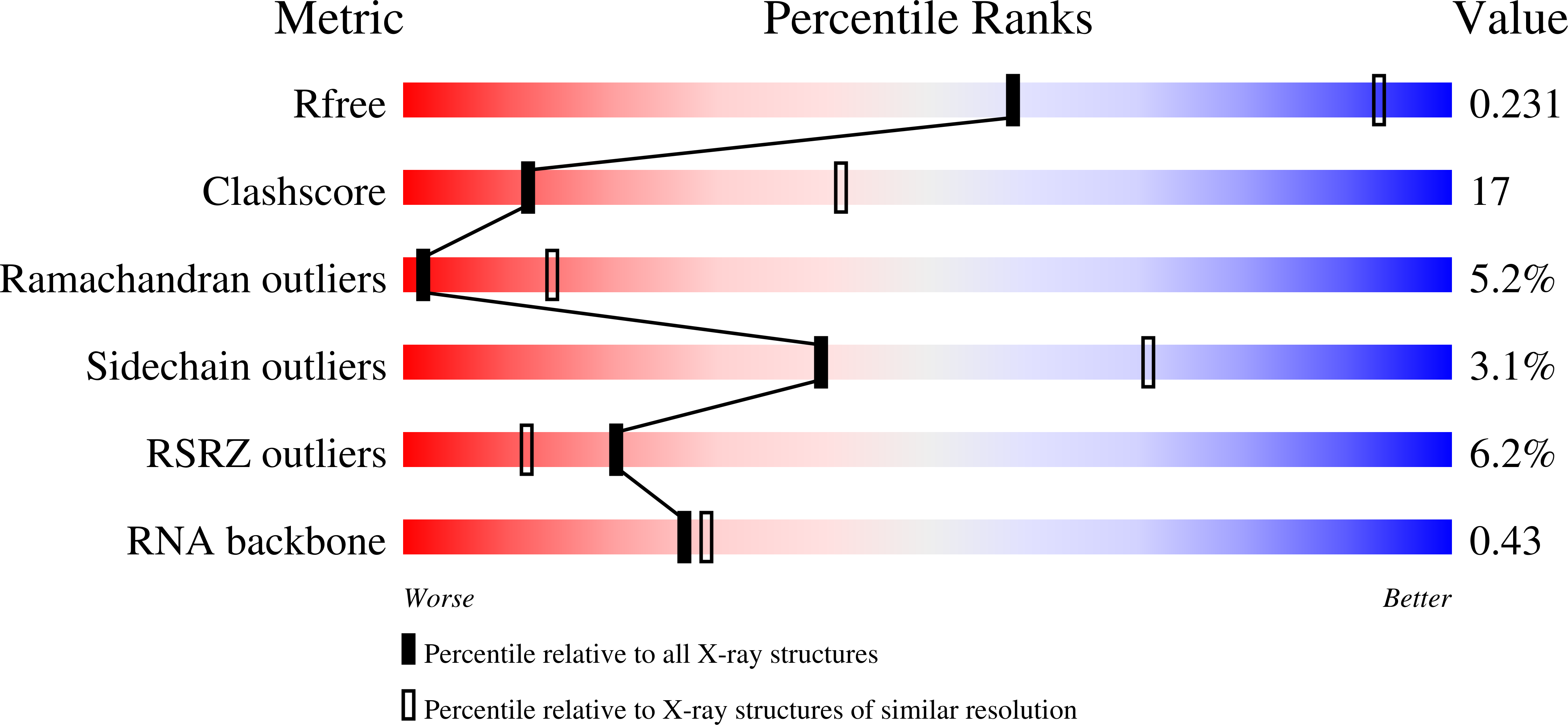
Resolution:
3.22 Å
R-Value Free:
0.25
R-Value Work:
0.22
R-Value Observed:
0.22
Space Group:
I 2 2 2