Deposition Date
2020-08-04
Release Date
2021-08-18
Last Version Date
2024-01-31
Entry Detail
PDB ID:
6ZZN
Keywords:
Title:
Crystal structure of the cubic catalytic core of the Mycobacterium tuberculosis branched-chain alphaketoacid acyltransferase component (E2b).
Biological Source:
Source Organism:
Mycobacterium tuberculosis H37Rv (Taxon ID: 83332)
Host Organism:
Method Details:
Experimental Method:
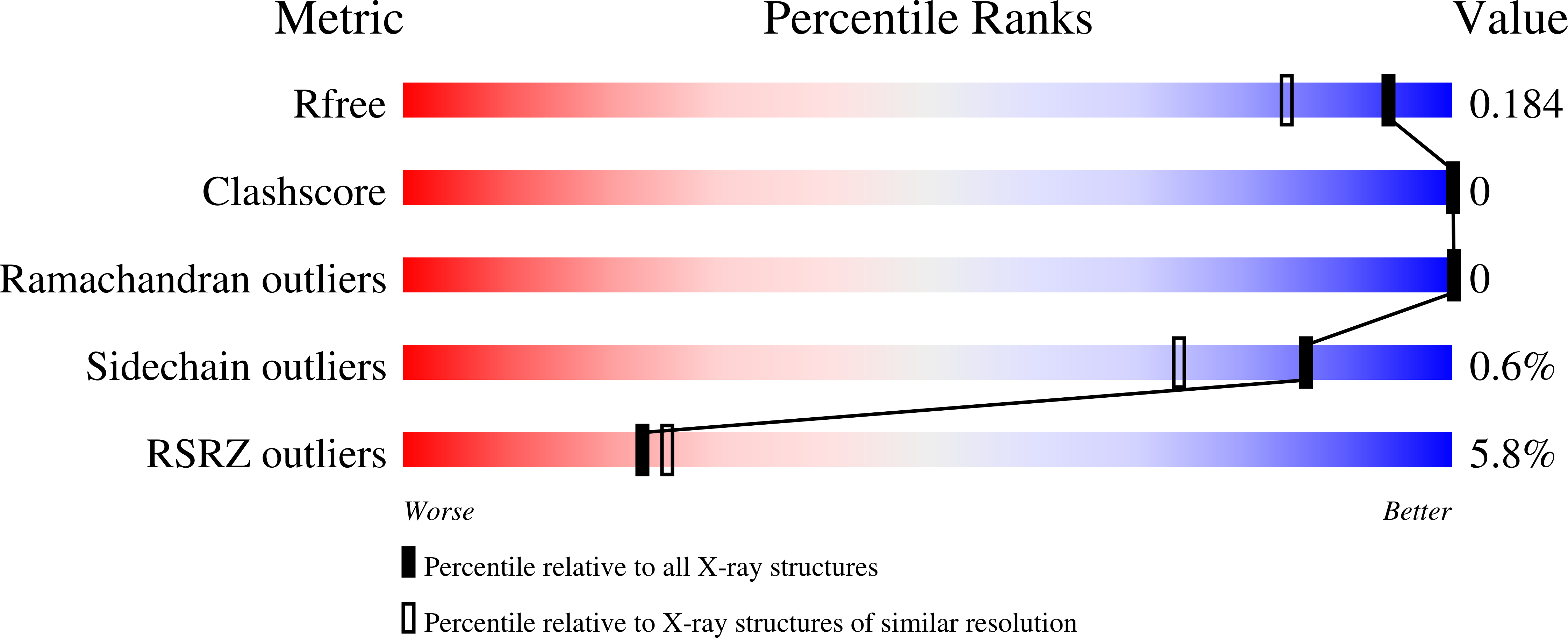
Resolution:
1.50 Å
R-Value Free:
0.19
R-Value Work:
0.18
R-Value Observed:
0.18
Space Group:
F 4 3 2