Deposition Date
2020-08-04
Release Date
2021-08-18
Last Version Date
2024-01-31
Entry Detail
PDB ID:
6ZZL
Keywords:
Title:
Crystal structure of the catalytic domain plus N-terminal linker of the acetyltransferase AceF (E2p) from Corynebacterium glutamicum.
Biological Source:
Source Organism:
Host Organism:
Method Details:
Experimental Method:
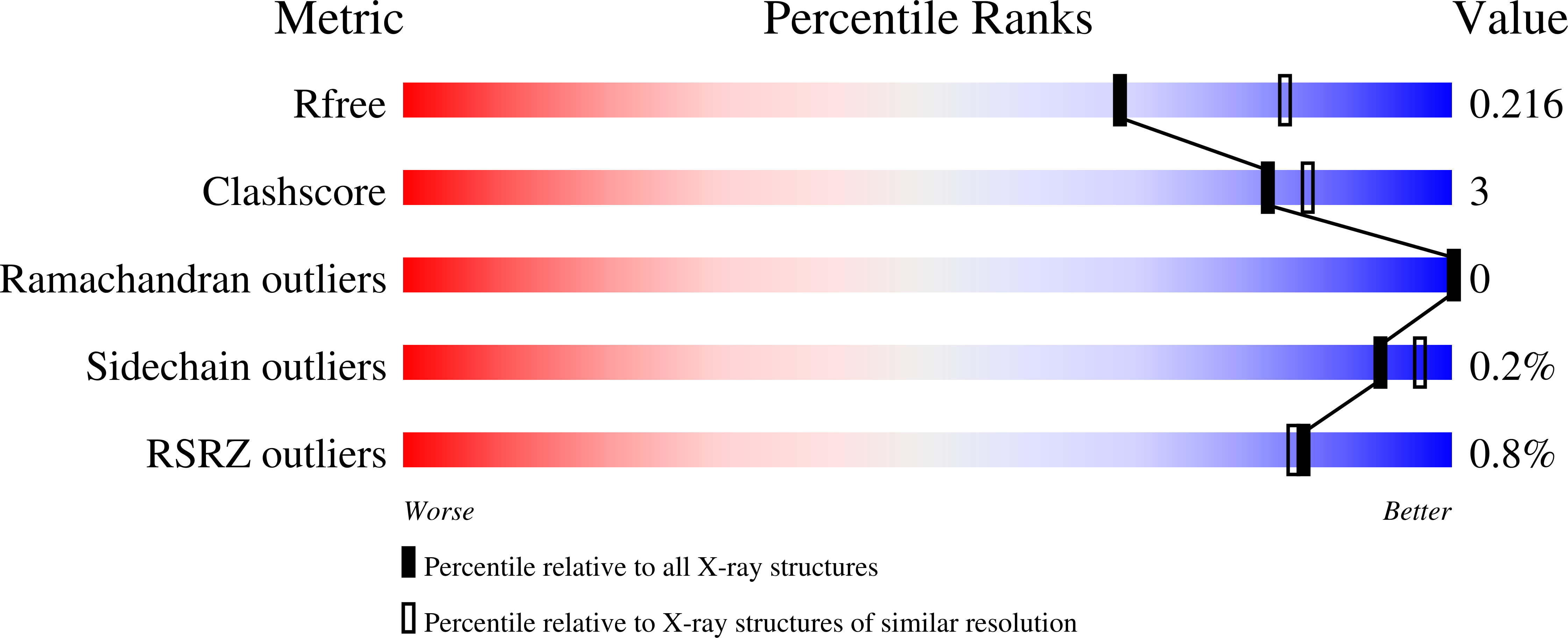
Resolution:
2.23 Å
R-Value Free:
0.21
R-Value Work:
0.19
R-Value Observed:
0.19
Space Group:
P 64