Abstact
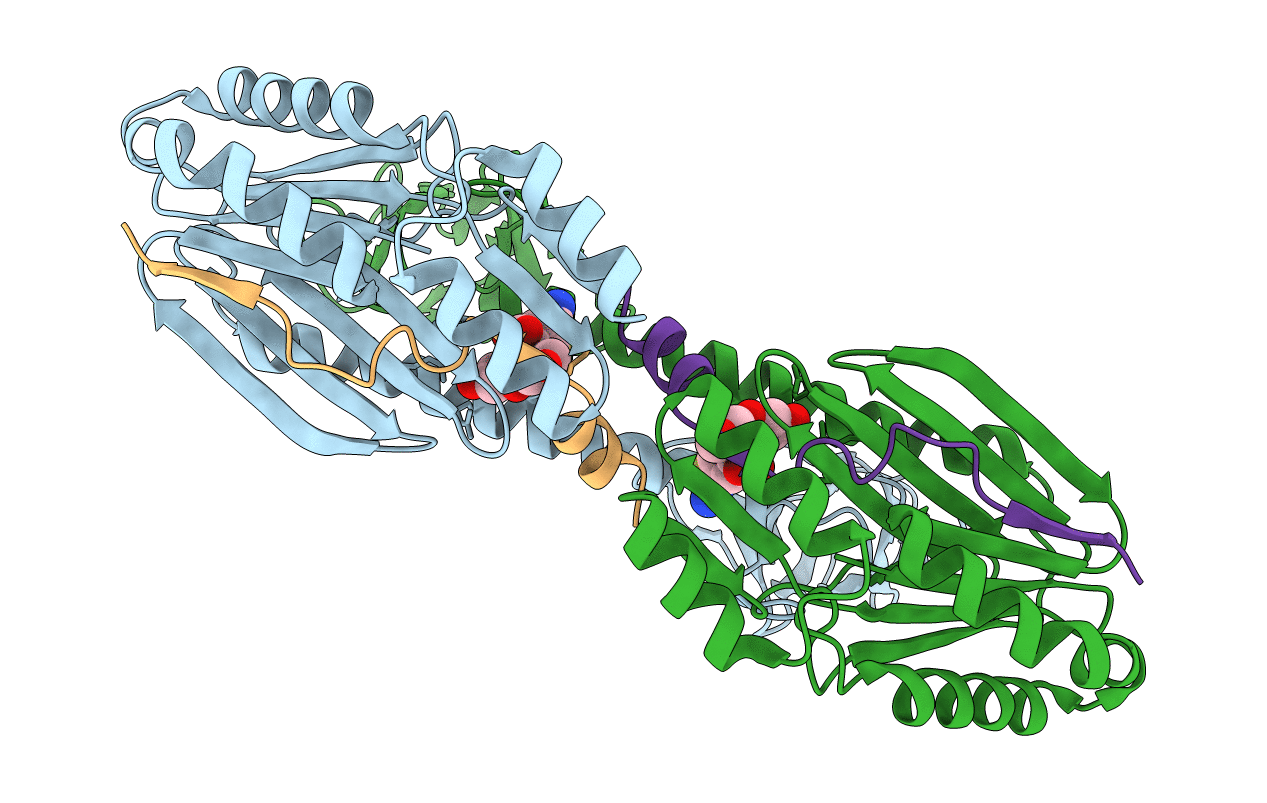
Sequence-specific protein ligations are widely used to produce customized proteins "on demand." Such chimeric, immobilized, fluorophore-conjugated or segmentally labeled proteins are generated using a range of chemical, (split) intein, split domain, or enzymatic methods. Where short ligation motifs and good chemoselectivity are required, ligase enzymes are often chosen, although they have a number of disadvantages, for example poor catalytic efficiency, low substrate specificity, and side reactions. Here, we describe a sequence-specific protein ligase with more favorable characteristics. This ligase, Connectase, is a monomeric homolog of 20S proteasome subunits in methanogenic archaea. In pulldown experiments with Methanosarcina mazei cell extract, we identify a physiological substrate in methyltransferase A (MtrA), a key enzyme of archaeal methanogenesis. Using microscale thermophoresis and X-ray crystallography, we show that only a short sequence of about 20 residues derived from MtrA and containing a highly conserved KDPGA motif is required for this high-affinity interaction. Finally, in quantitative activity assays, we demonstrate that this recognition tag can be repurposed to allow the ligation of two unrelated proteins. Connectase catalyzes such ligations at substantially higher rates, with higher yields, but without detectable side reactions when compared with a reference enzyme. It thus presents an attractive tool for the development of new methods, for example in the preparation of selectively labeled proteins for NMR, the covalent and geometrically defined attachment of proteins on surfaces for cryo-electron microscopy, or the generation of multispecific antibodies.