Deposition Date
2020-07-24
Release Date
2020-11-25
Last Version Date
2024-05-01
Entry Detail
PDB ID:
6ZV9
Keywords:
Title:
Terbium(III)-bound de novo TIM barrel-ferredoxin fold fusion dimer with 4-glutamate binding site and tryptophan antenna (TFD-EE N6W)
Biological Source:
Source Organism(s):
synthetic construct (Taxon ID: 32630)
Expression System(s):
Method Details:
Experimental Method:
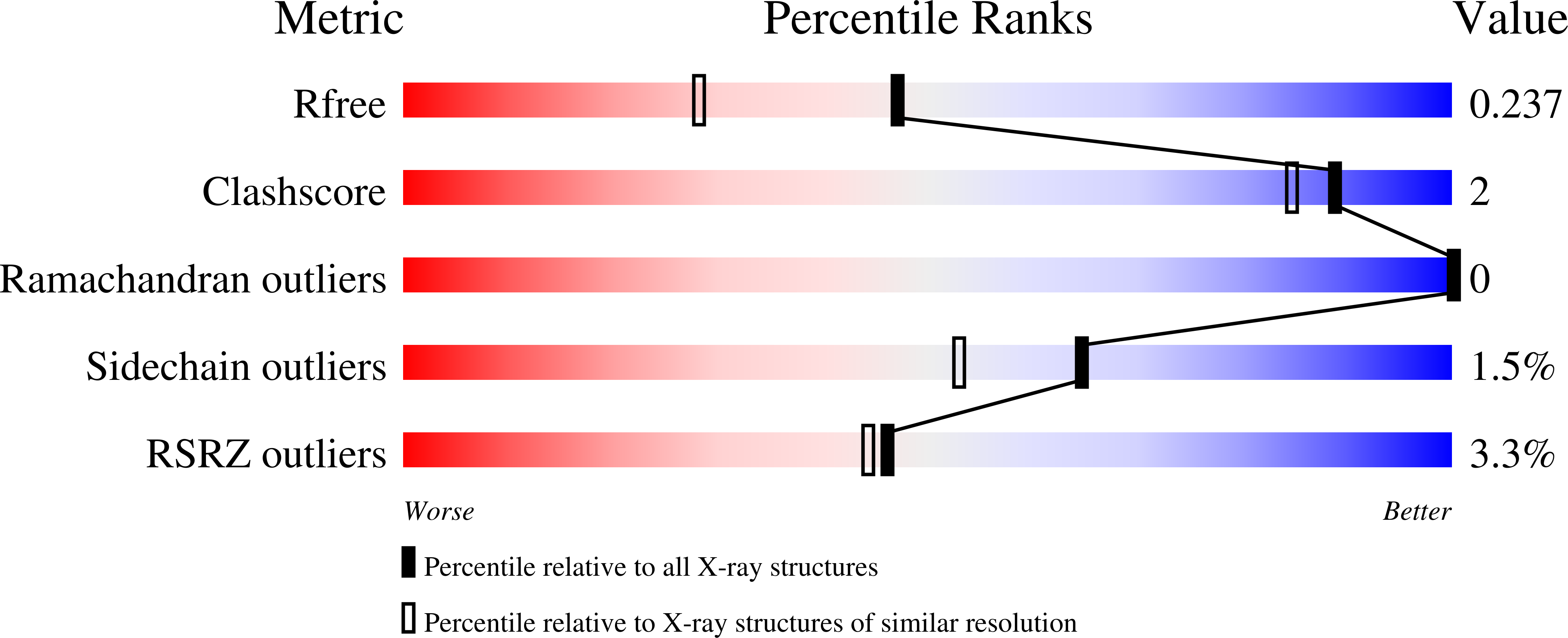
Resolution:
1.85 Å
R-Value Free:
0.22
R-Value Work:
0.19
R-Value Observed:
0.19
Space Group:
P 1 21 1