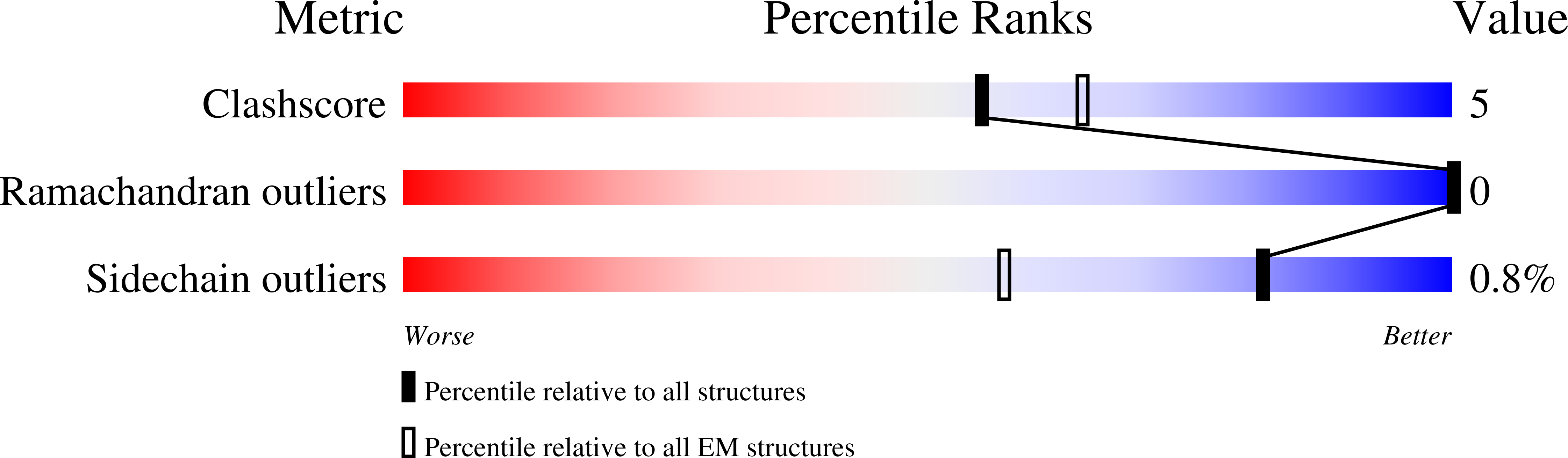
Deposition Date
2020-07-08
Release Date
2020-07-22
Last Version Date
2024-10-16
Entry Detail
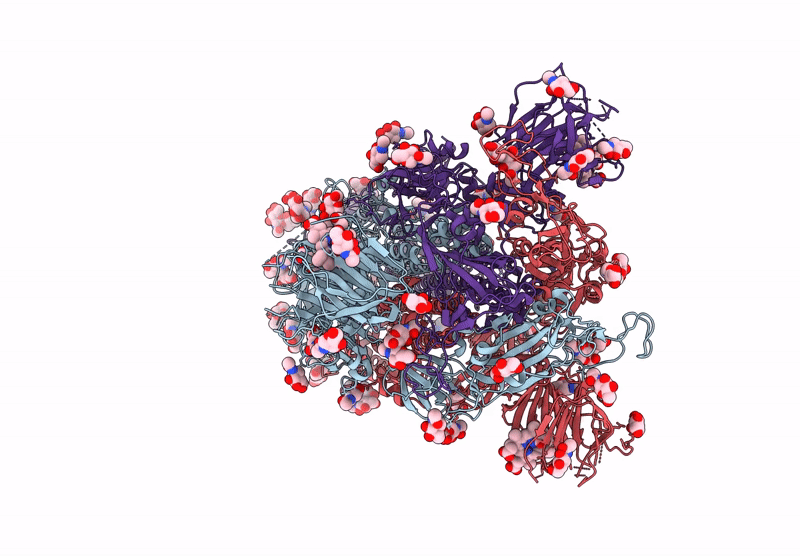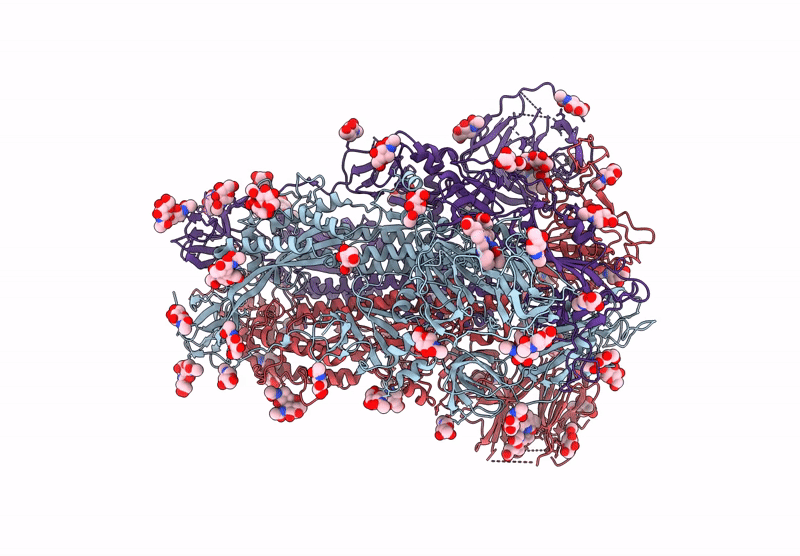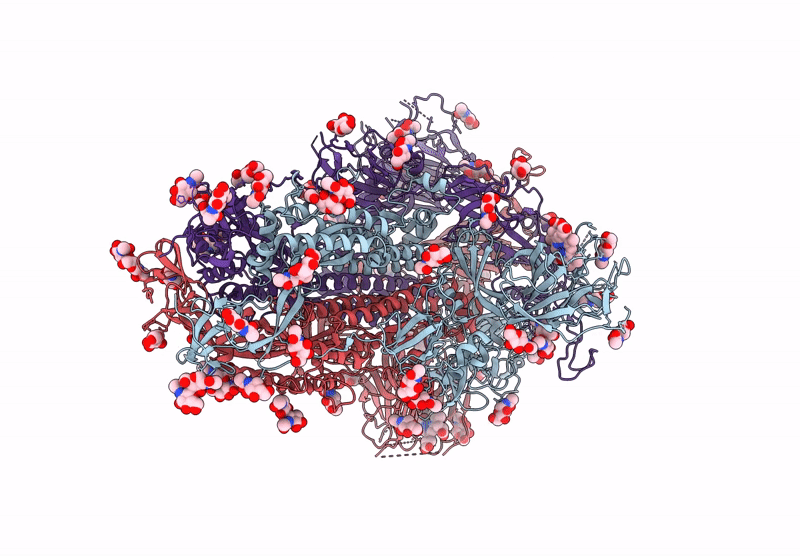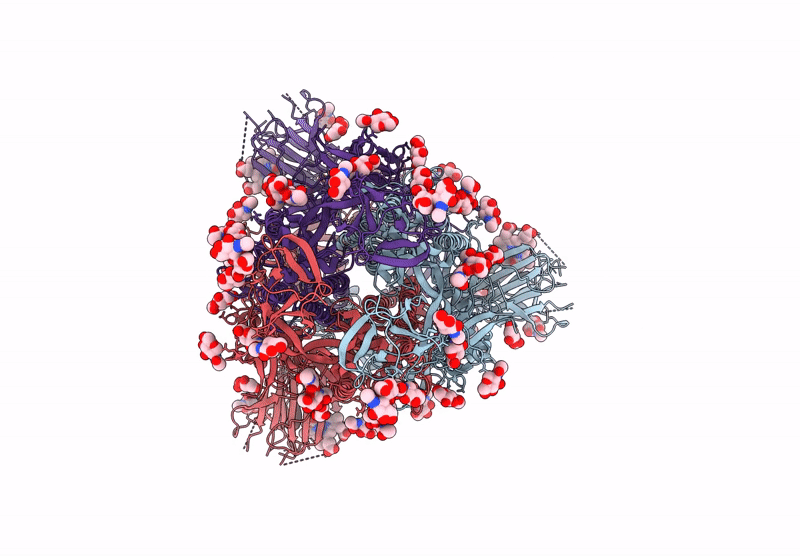
PDB ID:
6ZOZ
Keywords:
Title:
Structure of Disulphide-stabilized SARS-CoV-2 Spike Protein Trimer (x1 disulphide-bond mutant, S383C, D985C, K986P, V987P, single Arg S1/S2 cleavage site) in Locked State
Biological Source:
Source Organism:
Host Organism:
Method Details:
Experimental Method:
Resolution:
3.50 Å
Aggregation State:
PARTICLE
Reconstruction Method:
SINGLE PARTICLE