Deposition Date
2020-06-24
Release Date
2020-12-09
Last Version Date
2024-10-23
Entry Detail
PDB ID:
6ZI4
Keywords:
Title:
Ultrafast Structural Response to Charge Redistribution Within a Photosynthetic Reaction Centre - 5 ps (a) structure
Biological Source:
Source Organism(s):
Blastochloris viridis (Taxon ID: 1079)
Method Details:
Experimental Method:
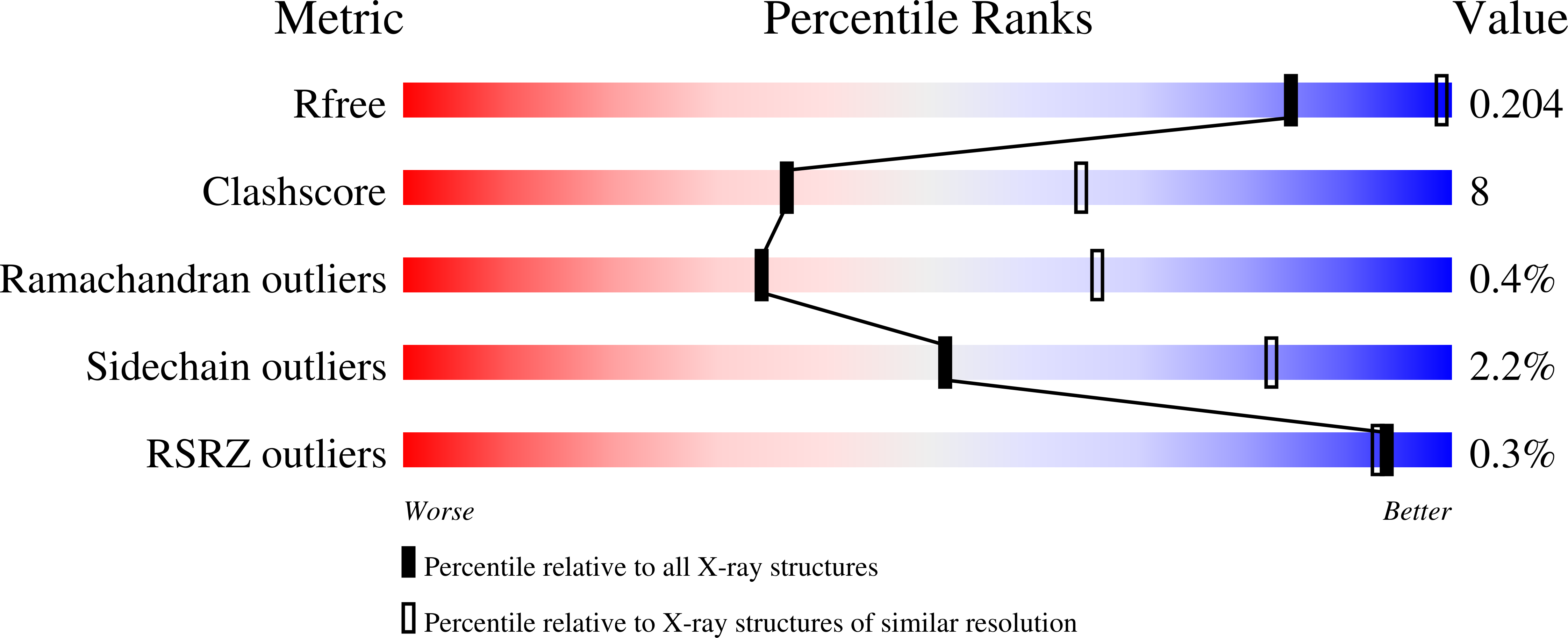
Resolution:
2.80 Å
R-Value Free:
0.20
R-Value Work:
0.17
R-Value Observed:
0.17
Space Group:
P 43 21 2