Deposition Date
2020-06-19
Release Date
2020-09-16
Last Version Date
2024-05-15
Entry Detail
PDB ID:
6ZGQ
Keywords:
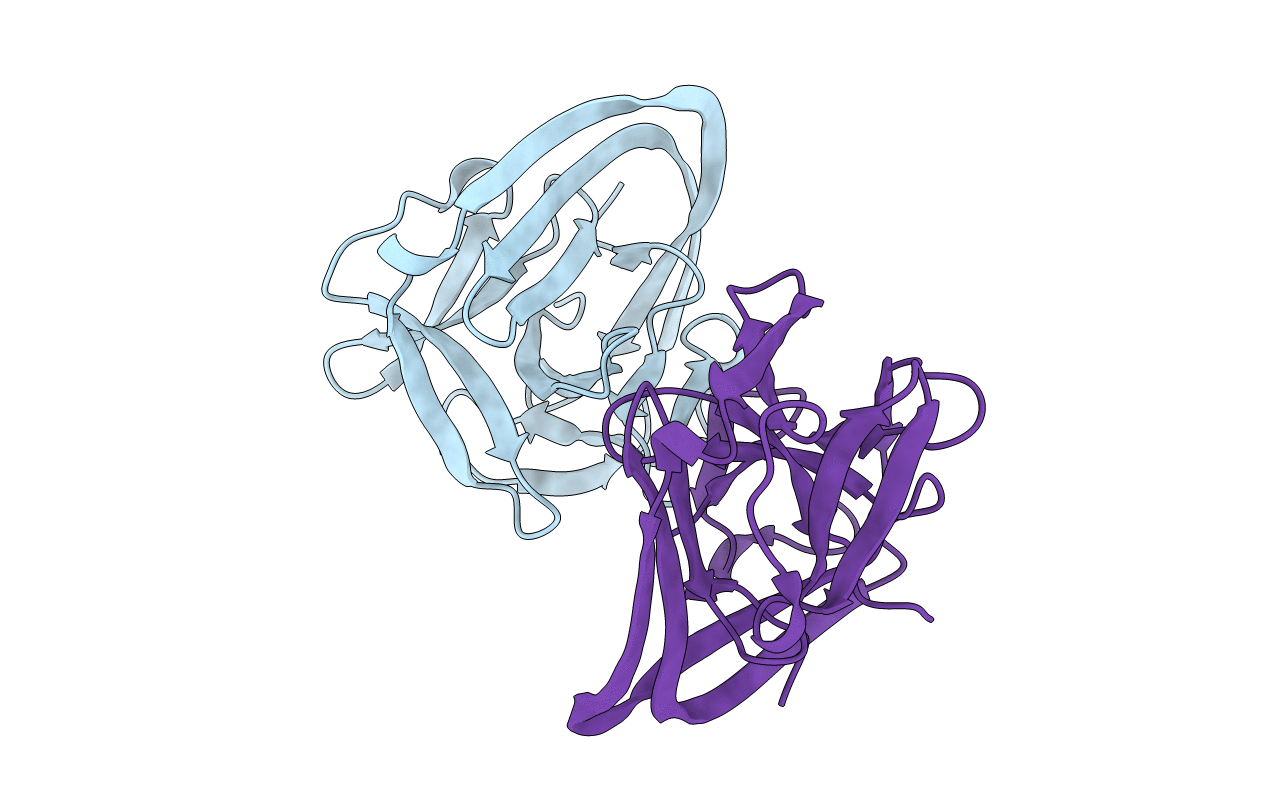
Title:
AceL NrdHF class 3 split intein GSH linked splice inactive variant - C124A, N146A
Biological Source:
Source Organism:
metagenome (Taxon ID: 256318)
Host Organism:
Method Details:
Experimental Method:
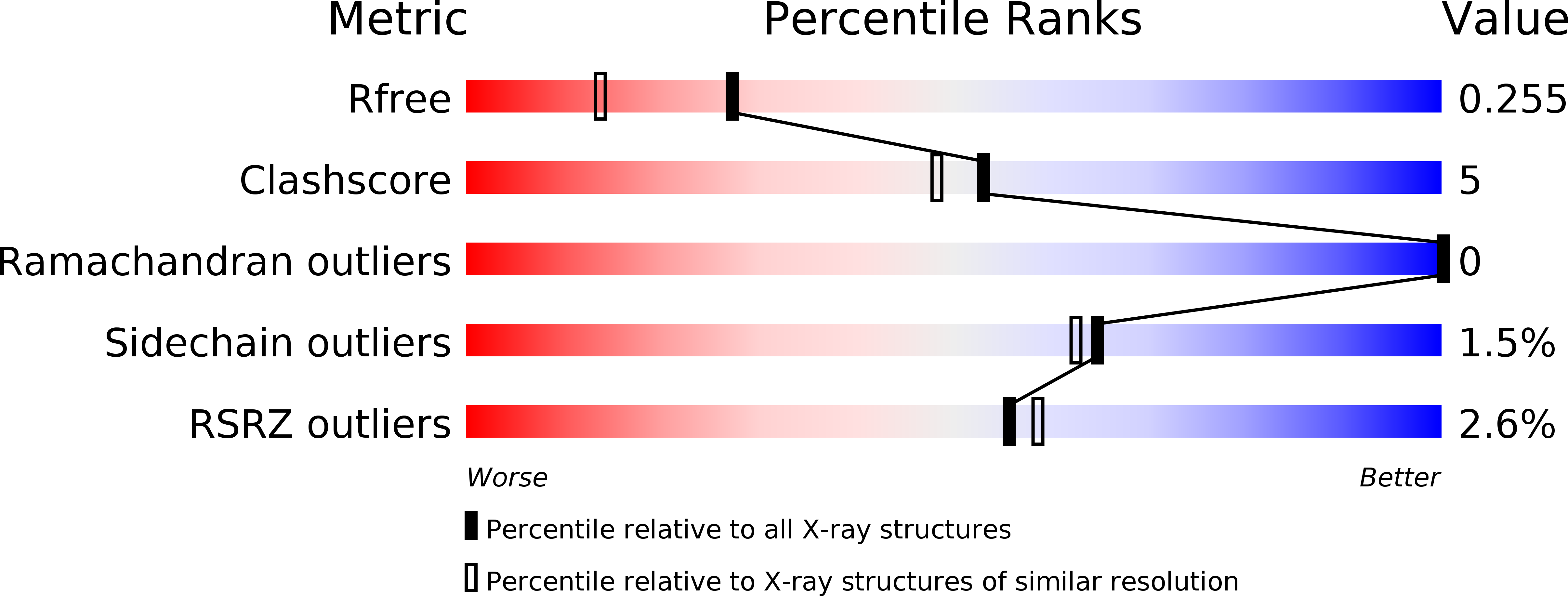
Resolution:
1.90 Å
R-Value Free:
0.25
R-Value Work:
0.22
R-Value Observed:
0.22
Space Group:
P 1