Deposition Date
2020-06-05
Release Date
2021-01-20
Last Version Date
2024-01-24
Entry Detail
PDB ID:
6ZAX
Keywords:
Title:
Nitrite-bound copper nitrite reductase from Bradyrhizobium sp. ORS 375 (two-domain) at low dose (0.5 MGy)
Biological Source:
Source Organism(s):
Bradyrhizobium sp. (strain ORS 375) (Taxon ID: 566679)
Expression System(s):
Method Details:
Experimental Method:
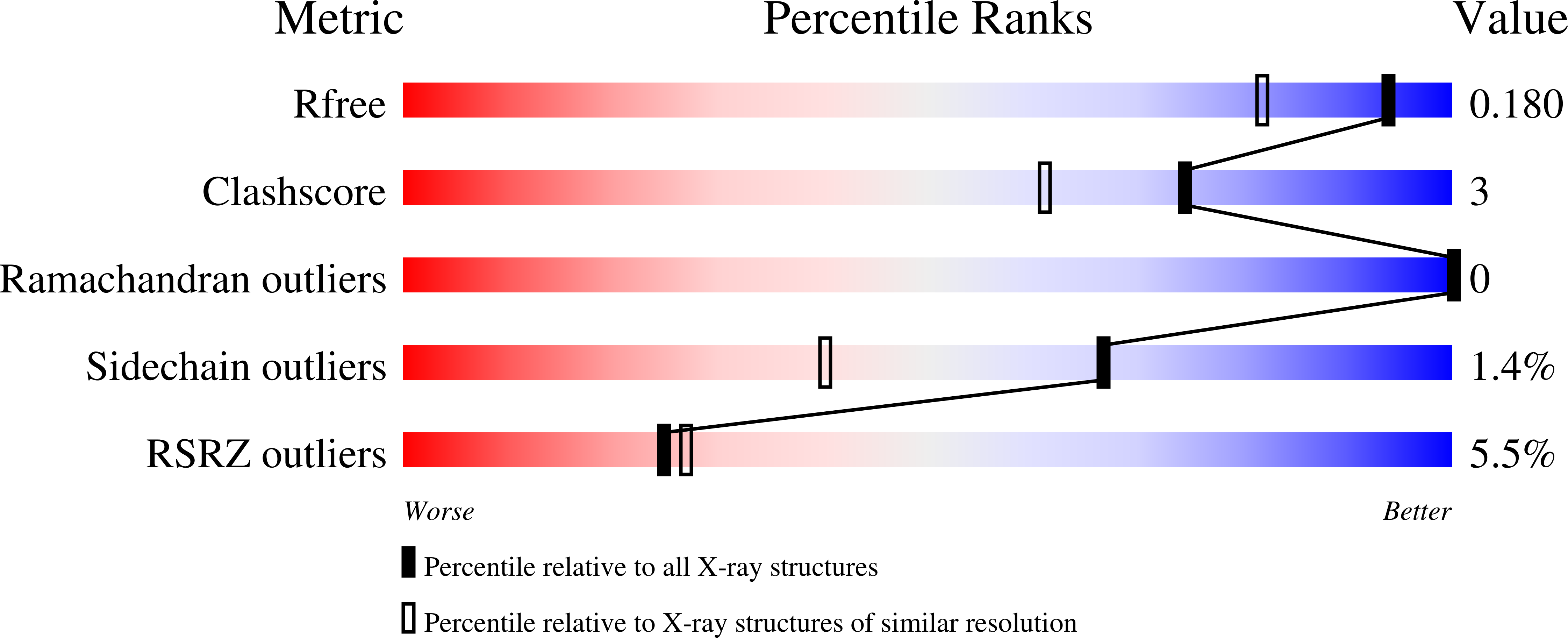
Resolution:
1.48 Å
R-Value Free:
0.17
R-Value Work:
0.14
R-Value Observed:
0.14
Space Group:
P 21 3