Deposition Date
2020-06-02
Release Date
2021-01-27
Last Version Date
2024-10-09
Entry Detail
PDB ID:
6Z8W
Keywords:
Title:
X-ray structure of the complex between human alpha thrombin and a thrombin binding aptamer variant (TBA-3G), which contains 1-beta-D-glucopyranosyl residue in the side chain of Thy3 at N3.
Biological Source:
Source Organism(s):
synthetic construct (Taxon ID: 32630)
Homo sapiens (Taxon ID: 9606)
Homo sapiens (Taxon ID: 9606)
Method Details:
Experimental Method:
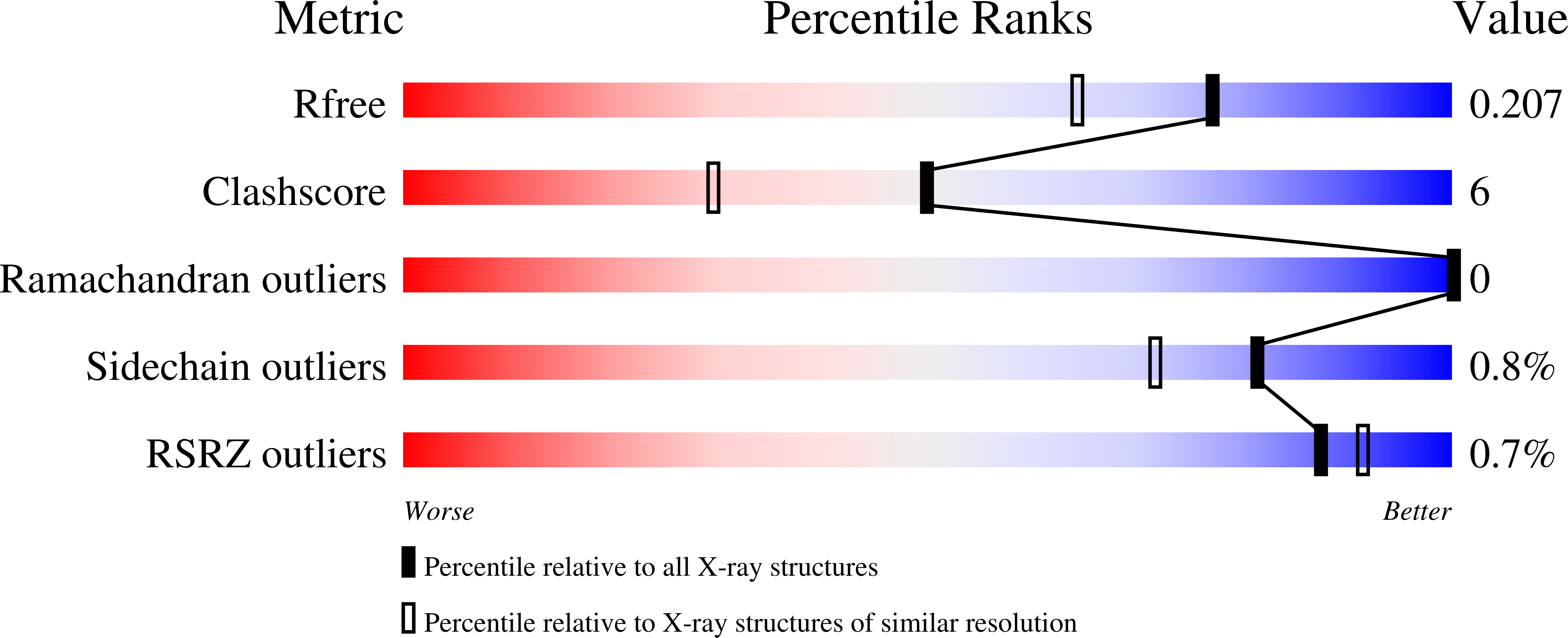
Resolution:
1.73 Å
R-Value Free:
0.20
R-Value Work:
0.17
R-Value Observed:
0.17
Space Group:
P 32 2 1