Deposition Date
2020-05-25
Release Date
2021-02-10
Last Version Date
2024-11-06
Entry Detail
PDB ID:
6Z4S
Keywords:
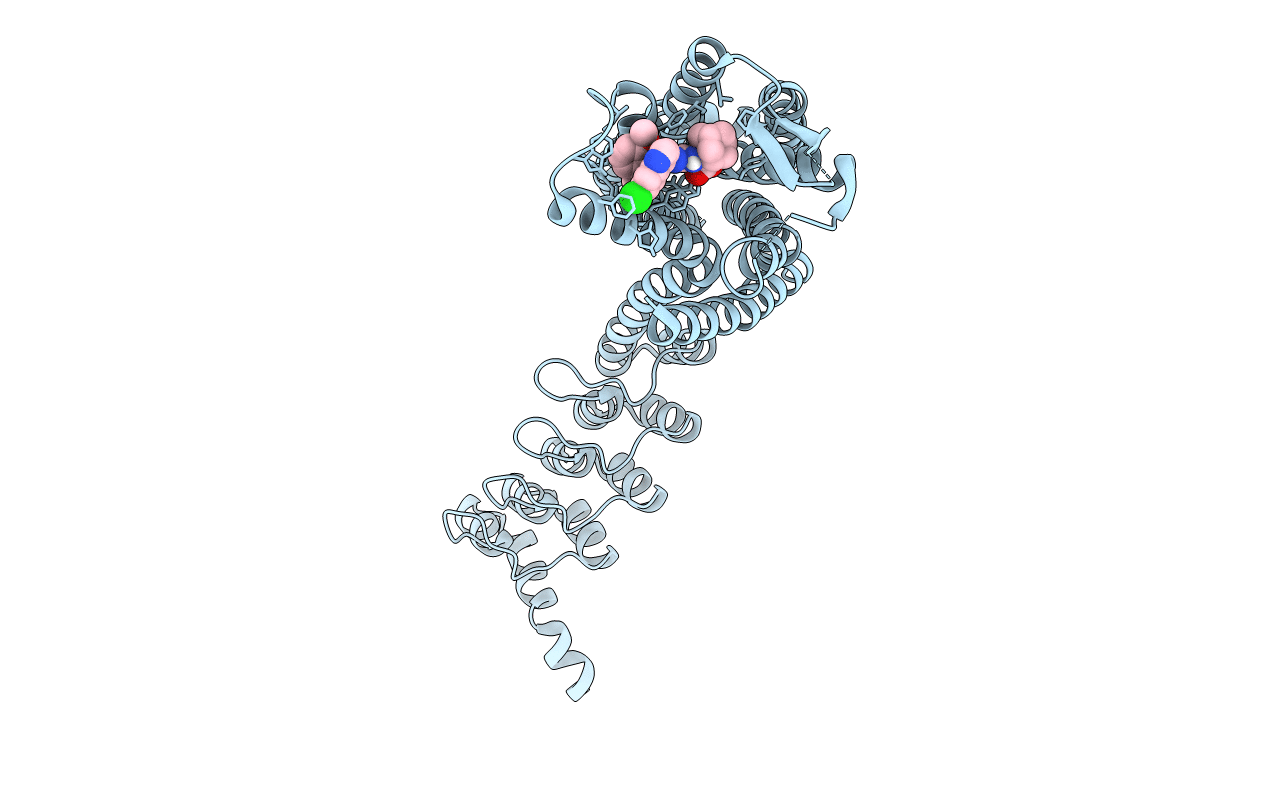
Title:
Crystal structure of the neurotensin receptor 1 (NTSR1-H4bmx) in complex with the small molecule inverse agonist SR48692
Biological Source:
Source Organism(s):
Rattus norvegicus (Taxon ID: 10116)
synthetic construct (Taxon ID: 32630)
synthetic construct (Taxon ID: 32630)
Expression System(s):
Method Details:
Experimental Method:
Resolution:
2.71 Å
R-Value Free:
0.28
R-Value Work:
0.27
Space Group:
P 21 21 21


