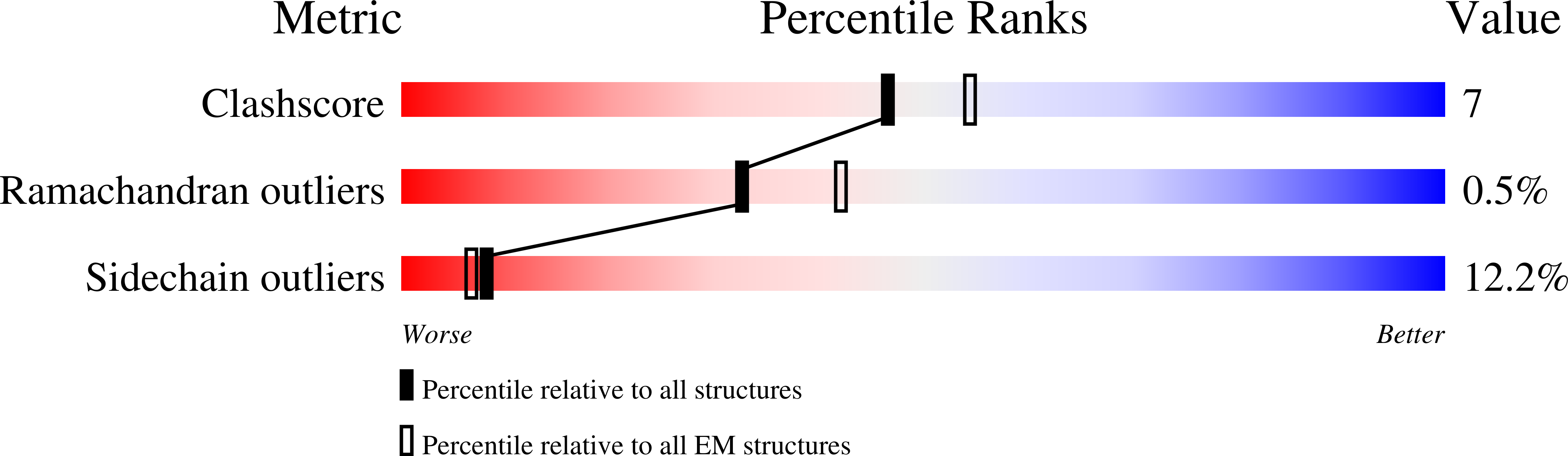Deposition Date
2020-04-20
Release Date
2020-06-24
Last Version Date
2024-05-22
Entry Detail
PDB ID:
6YRK
Keywords:
Title:
P140-P110 complex fitted into the cryo-electron density map of the heterodimer
Biological Source:
Source Organism(s):
Expression System(s):
Method Details:
Experimental Method:
Resolution:
4.10 Å
Aggregation State:
PARTICLE
Reconstruction Method:
SINGLE PARTICLE