Deposition Date
2020-04-15
Release Date
2020-11-25
Last Version Date
2025-10-01
Entry Detail
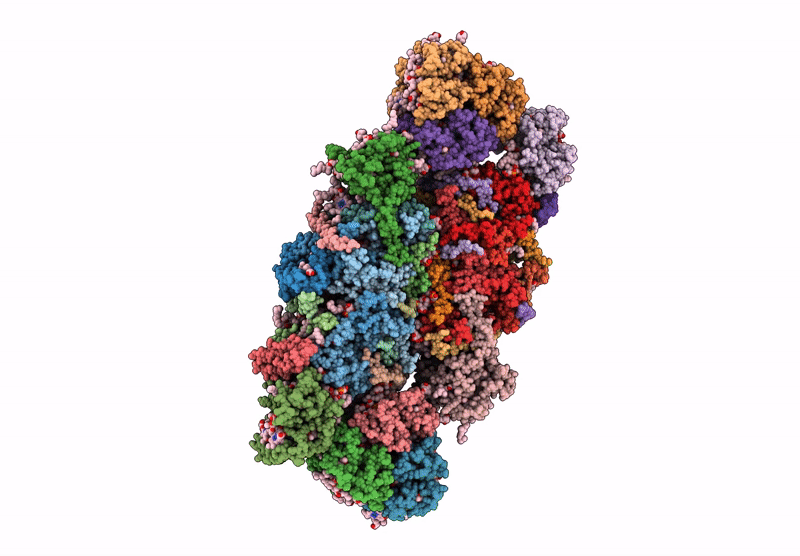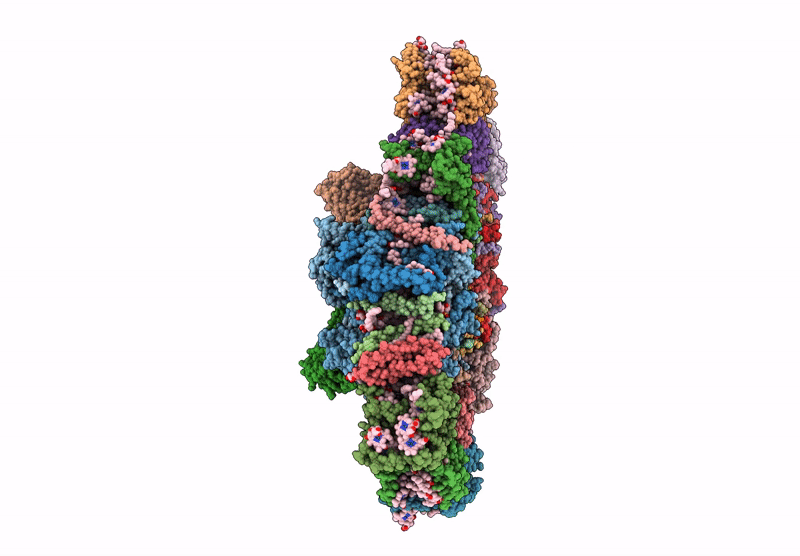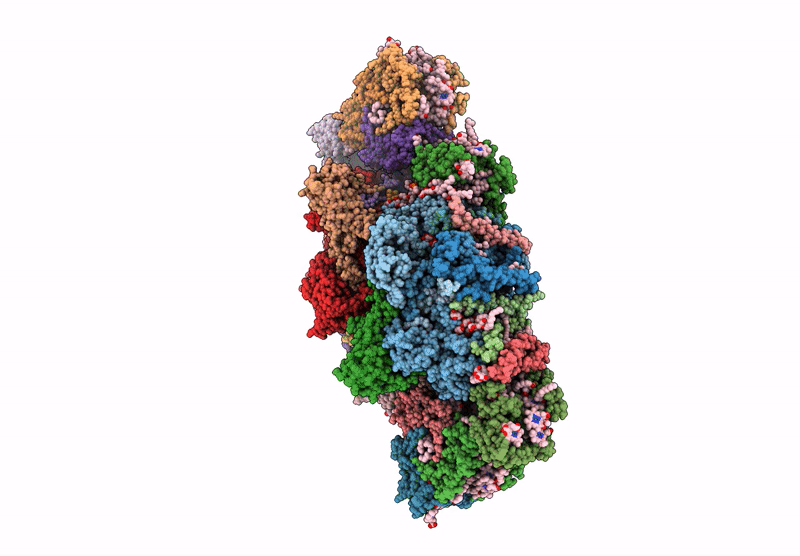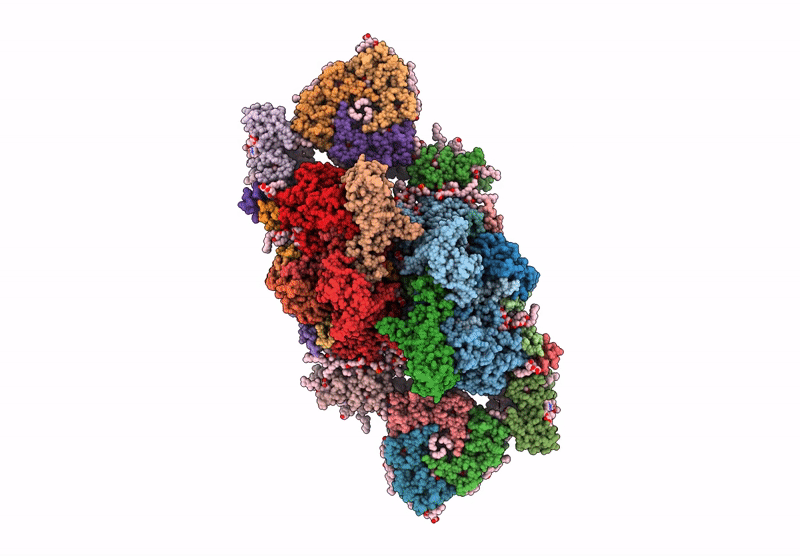
PDB ID:
6YP7
Keywords:
Title:
PSII-LHCII C2S2 supercomplex from Pisum sativum grown in high light conditions
Biological Source:
Source Organism(s):
Pisum sativum (Taxon ID: 3888)
Expression System(s):
Method Details:
Experimental Method:
Resolution:
3.80 Å
Aggregation State:
PARTICLE
Reconstruction Method:
SINGLE PARTICLE


